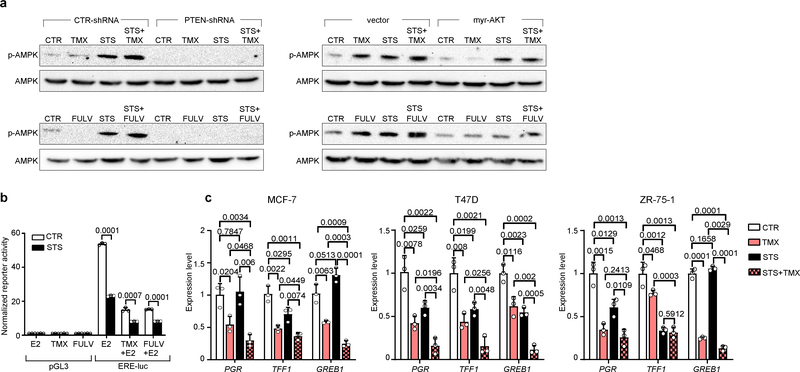
Extended Data Fig. 6 |. STS conditions cooperate with ET to activate AMPK and to dampen oestrogen receptor transcriptional activity in BC cells.
a, MCF7 cells transduced with a control vector, a PTEN-targeting shRNA or myr-AKT were plated into 6-well plates and cultured with or without STS conditions, TMX (5 μM), FULV (10 μM) or their combinations for 48 h. Afterwards, cells were subjected to protein lysate generation, and total and phosphorylated AMPK (Thr172) were detected by immunoblotting. The loading control for the immunoblots with PTEN-silenced MCF7 cells (vinculin) is shown in Supplementary Fig. 1. The loading control for the immunoblots with myr-AKT-expressing MCF7 (GAPDH) is shown in Extended Data Fig. 5j. Both loading controls were run on the same gels that were used to detect total and phosphorylated AMPK. For gel source data, see Supplementary Fig. 1. b, MCF7 cells were plated into 24-well plates and transfected with a pGL3 promoter plasmid or with a pS2/TFF1 reporter vector containing 1.3 kb of the proximal promoter of the oestrogen-responsive gene TFF1 cloned in the pGL3-basic backbone. Afterwards, cells were cultured with or without STS, TMX (5 μM), FULV (10 μM) or their combinations for 48 h. Finally, luciferase reporter activity was measured. c, MCF7, T47D and ZR-75–1 cells were plated in 6-well plates and cultured with or without STS, TMX (5 μM) or their combinations for 48 h. Thereafter, cells were subjected to RNA extraction, and PGR, TFF1 and GREB1 expression was detected by qPCR. In a–c, one representative experiment out of three is presented. In b, c, data are from biological replicates and are presented as mean ± s.d. P values were determined by two-tailed Student’s t-test.