Fig. 1.
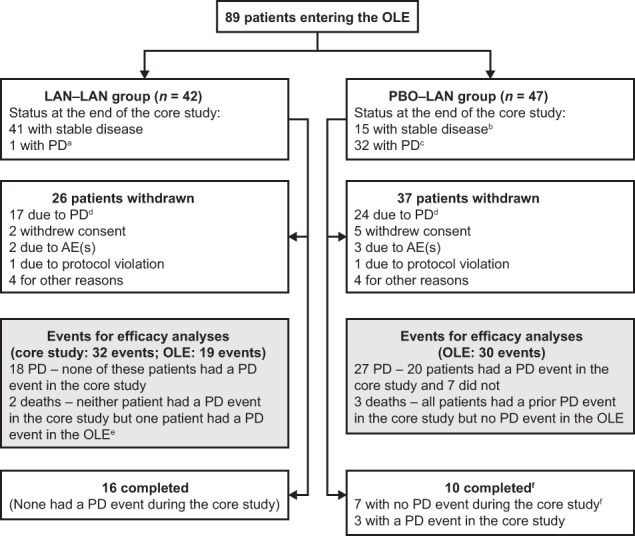
Flow of patients through the OLE. ‘Completed’ means the patient had had no PD events during the OLE at the time that the OLE was terminated. aOne patient was enroled by the investigator before centrally assessed PD was confirmed (the patient was withdrawn when the confirmation was received). bIncludes 1 patient who was withdrawn from the core study due to investigator judgement of PD but had an a posteriori central assessment of SD—this patient was then enroled in the OLE. cIncluding 1 patient who was withdrawn from the core study due to a centrally assessed PD, but was erroneously classified as having SD at the time of the core study database lock—this subject was censored in the primary analysis of PFS in the core study, but was included as an event in the analysis of PFS in the OLE. dWithdrawal from the OLE due to ‘PD’ did not always represent an event in the analysis of PFS: part-way through the study, the sponsor sent additional clarification to all sites on how to complete local tumour evaluations in order to have a standardised approach for the assessment of progression status across the study sites; all radiological scans that had already been evaluated were re-evaluated at this time. eOnly the PD event was included in the analysis of PFS. fIncludes 1 patient with PD at the final study visit. OLE open-label extension, LAN–LAN group patients receiving lanreotide autogel/depot in core study as well as the OLE study, PBO–LAN group patients receiving placebo in the core study before crossing over to lanreotide in the OLE study, AE adverse event, SD stable disease, PFS progression-free survival
