Fig. 2.
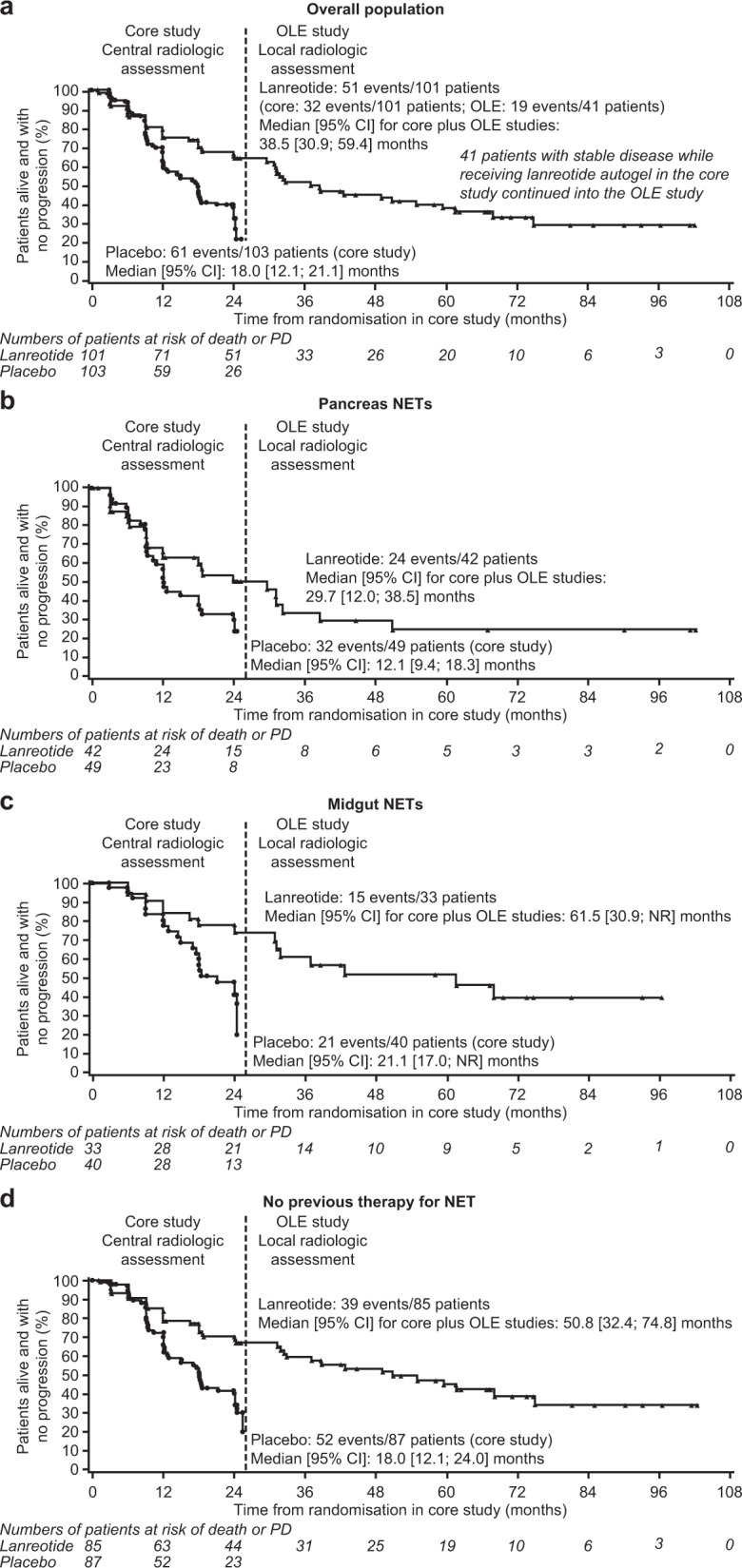
PFS for lanreotide autogel/depot from the CLARINET core study and the OLE and PFS for placebo from the core study: overall (a) and for subgroups according to primary tumour origin (b, c) and prior therapy (d). Events were PD (according to RECIST version 1.0) or death. Data are from the intention-to-treat population with months approximated based on 4 weeks per month. Core-study data are from all patients randomly allocated to double-blind treatment (lanreotide autogel/depot or placebo). The OLE data are only for patients originally randomly allocated to lanreotide in the core study who then continued into the OLE. The PFS data previously reported for placebo were based on 60 events at the time of database lock in the core study [1]; however, one patient with PD had been erroneously reported as having centrally assessed SD. This additional event been included in the analysis of the OLE data. For the pancreas and midgut data, primary tumour type is the basis for the analyses and results are based on the combination of the various primary tumour locations. OLE open-label extension, PD progressive disease, NET neuroendocrine tumour, NR not reached, PFS progression-free survival, RECIST Response Evaluation Criteria In Solid Tumours, SD stable disease
