Figure 1.
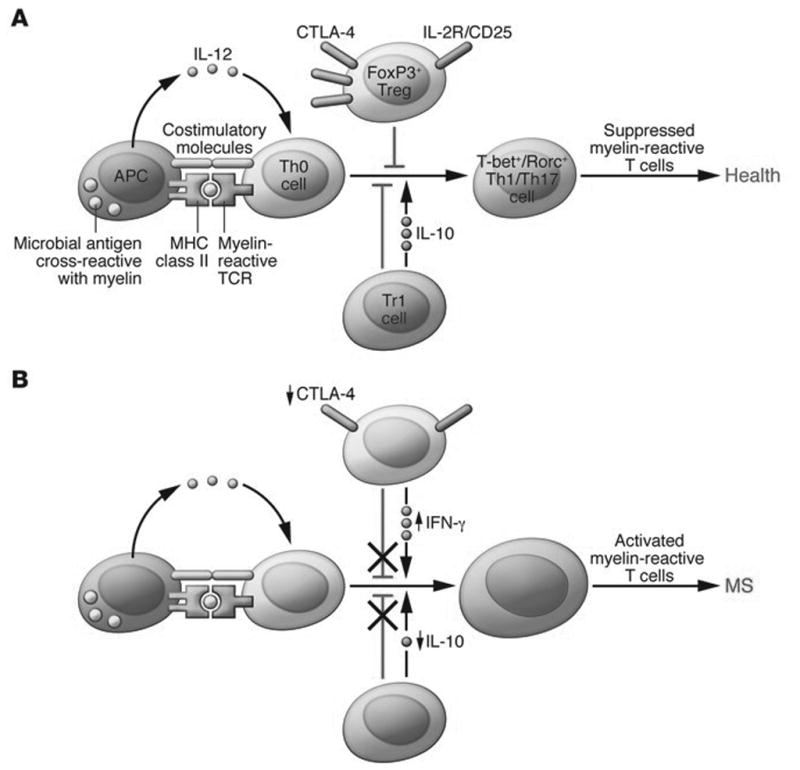
Defects in peripheral immune regulation lower the activation barrier for autoreactive T cells. (A) In normal homeostasis, APCs digest microbial antigens or self proteins and present them to naive T cells in the context of co-stimulatory molecules. An appropriate cytokine milieu can drive differentiation of these naive autoreactive T cells to a Th1 or Th17 cell phenotype; however, these potentially pathogenic T cells are not activated due to the actions of peripheral regulatory immune cell populations, such as FoxP3+ Tregs and Tr1 cells. Via the actions of co-inhibitory molecules and cytokines such as IL-10 and TGF-β, autoreactive T cells become anergic and autoimmune disease is prevented. Other mechanisms, such as thymic deletion and lack of co-stimulatory molecules on APCs, are also involved in controlling autoreactive T cells. (B) MS patients have defects in peripheral immune regulation, including higher expression of co-stimulatory molecules on APCs, lower CTLA-4 levels, and lower IL-10 production. Additionally, MS patients have an increased frequency of IFN-γ–secreting Tregs relative to healthy controls. Thus, the barrier for activation of autoreactive T cells is lowered for MS patients. Activated myelin-reactive T cells can then adhere to and extravasate across the choroid plexus and BBB, where they can initiate an inflammatory milieu that gives license to further waves of inflammation and eventual epitope spreading. Reproduced with permission from [1]
