Fig. 1. Study design and systematically coordinated tissue biopsy system.
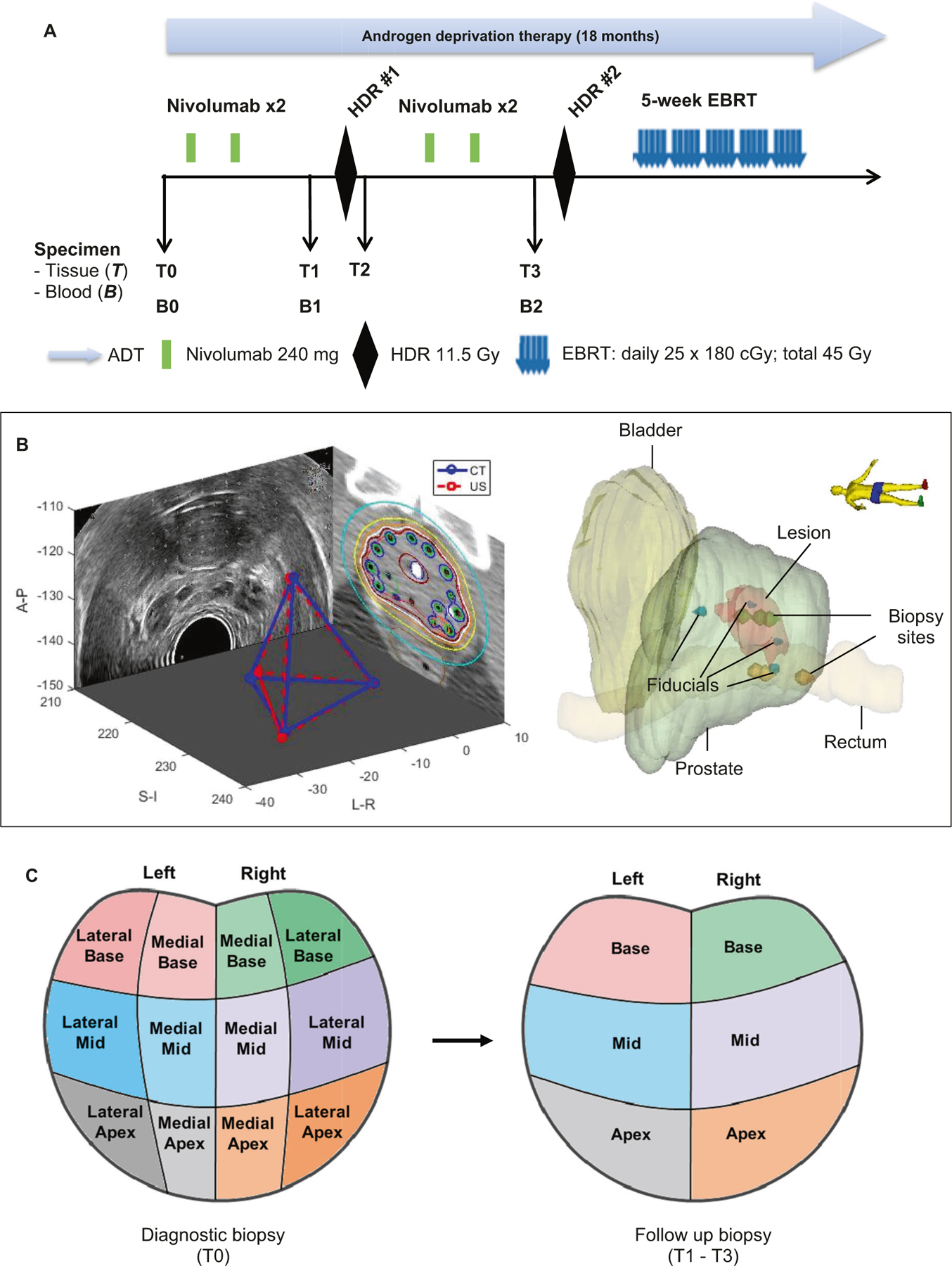
a Study schema. ADT: Androgen deprivation therapy. EBRT: External beam radiation therapy. HDR: High dose brachytherapy. Nivo: nivolumab. B0/T0: Baseline blood and diagnostic biopsy. B1/T1: Specimen collected after two cycles of nivolumab, and prior to HDR#1 brachytherapy. T2: Specimen collection within 2 h post HDR#1. B2/T3: Specimen collection after 4 cycles of nivolumab and four weeks after HDR#1, but prior to HDR#2. b Tissue collection schema. A carefully designed coordinates system was used to determine the location of the 6-core biopsy sites (left apex, left mid, left base, right apex, right mid, and right base). Four fiducial markers were placed into the apex, base, left mid and right mid gland. Biopsies were performed under ultrasound guidance by combining the prostate template grid, the ultrasound transducer stepper, and the location of the fiducial marker. Mirada’s multimodality registration was used to rigidly register the MRI image to the planning CT using local registration. The biopsy sites were documented for follow-up biopsy reference. c Prostate biopsy locations. Left panel: T0 is diagnostic biopsy prior to treatment; Right panel: T1 is post-nivolumab (2 Cycles) and Pre high dose radiation (HDR-Brachytherapy) biopsy; T2 is within 2 h post-HDR brachytherapy, T3 is post-nivolumab (4 cycles) and 1 month post-HDR brachytherapy.
