Abstract
We investigated the effect of the strain Bacillus subtilis GM5 on growth, feed conversion, and the composition of cecum microbiota in broiler chickens. Half of which received a control diet, while the other half was fed a diet supplemented with GM5 spores. Cecal contents on days 1, 10, and 42 were subjected to metataxonomic analysis. Principal Component Analysis showed that the control and probiotic groups formed three separate clusters, indicating changes, which occurred gradually in microbial communities. On day 1, Firmicutes (53.87–57.61%) and Proteobacteria (43.77–38.93%) were prevalent in both groups, whereas samples of days 10 and 42 were predominantly occupied by Firmicutes (54.55–81.79%) and Bacteroidetes (26.94–30.45%). In the group of chickens treated with probiotic, the average daily gain in body weight was higher, while feed conversion decreased by 1.44%. A surge in the presence of beneficial bacteria of the Ruminococcaceae family was observed. The introduction of the probiotic led to an elevated Firmicutes/Bacteroidetes ratio, which positively correlated with chickens’ bodyweight (Spearman ρ = 1.0, P < 0.05). Supplementing broiler feed with B. subtilis GM5 spores leads to improved feed intake and digestibility, which is paramount in reducing the cost of the final product. Thus, the probiotic strain GM5 modulates the cecal microbiota of broiler chickens and increases microbial diversity, which is well exhibited on the 42nd day.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-020-02634-2.
Keywords: Broiler, Probiotics, GIT of chickens, Cecum, 16S rRNA gene, Metataxonomic
Introduction
Rapid growth in poultry production has been associated with the widespread use of antibiotic growth promoters (AGPs) aimed towards enhancing growth performance and inhibiting the spread of certain diseases (Musa et al. 2019). Side effects, such as the development and spread of antibiotic resistance and potentially harmful effects to the intestinal microbiota have stimulated the necessity in limiting the use of AGPs (Boeckel et al. 2015; Bai et al. 2017). Natural growth promoters (NGPs), such as prebiotics, phytobiotics, probiotics, or direct-fed microbial (DFM), are used as an alternative to antibiotics in animal husbandry (Huang et al. 2018; Musa et al. 2019).
The intestinal microbiota remains of great interest to researchers trying to improve the productivity and health of birds, as well as poultry food safety (Oakley et al. 2014a, b; Richards et al. 2019). The balanced dynamics of intestinal microbiota plays a vital role in the metabolic and immune processes of the organism of poultry birds (Rychlik 2020). The cecum of broilers shows the highest species diversity and this section of the GIT are still being investigated (Videnska et al. 2013; Medvecky et al. 2018; Hong et al. 2019). The primary function of the cecum involves the fermentation of nutrients, through which digestive processes in the cecum provide up to 10% of the metabolized energy in young and adult chicken (Clench and Mathias 1995; Jozefiak et al. 2004). In addition, the cecum is the hot site of colonization of pathogenic microorganisms (Crhanova et al. 2011). Monitoring the dynamics of microbiota helps to enhance the growth and productivity of birds, as well as adjust the diet using beneficial bacterial strains (Choi et al. 2015; Shang et al. 2018).
The formation of GI microbiota of commercially hatched birds occurs differently than in wild chickens since contact between parents and offspring are completely interrupted (Kubasova et al. 2019; Rychlik 2020). During the colonization of commercially hatched birds, the assembly of beneficial groups of bacteria could be delayed over time, which in turn could adversely affect the health and development of chickens. Furthermore, commercially hatched fowls are extremely sensitive to colonization by different pathogens such as Salmonella spp., Escherichia coli and Campylobacter spp. (Ranjitkar et al. 2016; Varmuzova et al. 2016). The composition of the intestinal microbiota is modulated by several external factors, including bird type and breed, sex, age, feed access, medication, antibiotics, stress, housing and other factors (Zhao et al. 2013; Antonissen et al. 2015; Schokker et al. 2015; Kers et al. 2018).
Bacillus spp. have gained increasing attention for industrial applications as probiotics. They form spores with resistance to the high temperatures, used in the modern production of feed for poultry, and show stability to low pH, bile and enzymes found in GIT of chickens (Ducatelle et al. 2015; Wealleans et al. 2017). Once in the intestines of the bird, Bacillus spores can germinate and produce secondary metabolites with potential health benefits for the host animal (Gao et al. 2017). Studies have indicated increased productivity in broiler and laying birds when introduced to Bacillus-based probiotics (Lee et al. 2015; Gadde et al. 2017; Rhayat et al. 2017; Neijat et al. 2018, 2019). Early stimulation of beneficial microbiota in broiler chickens is critical in enhancing productivity and health.
In previous studies, we showed that the Bacillus subtilis GM5 strain effectively inhibits the growth of pathogenic and opportunistic bacteria and phytopathogenic fungi, possesses probiotic properties, and can be used as a probiotic strain for broiler chickens (Mardanova et al. 2017; Khadieva et al. 2018). In addition, genes responsible for the synthesis of antimicrobial lipopeptides that can provide antagonistic activity against pathogens were annotated in the B. subtilis GM5 genome (Hadieva et al. 2019).
The objective of this study was to evaluate the effects of B. subtilis GM5 spores on the growth performance, nutrient utilization, and cecal microbial composition of broilers on the 1st, 10th and 42nd days of age.
Materials and methods
Bacterial strain
For bacterial spores acquisition, a 4-day old B. subtilis GM5 culture was incubated at 60 °C for 90 min to eliminate vegetative cells (Khadieva et al. 2018). The Bacillus subtilis GM5 strain is currently stored at − 80 °C and has been registered into the Collection of the Microbiology Department at the Institute of Fundamental Medicine and Biology of the Kazan Federal University (KFU) under the accession number GM RT 5. The probiotic was introduced into dry feed by spraying with a spray gun with constant manual stirring. The final product contained 1 × 107 spores/g of B. subtilis GM5.
Poultry farm, diets and experimental design
All experiments were carried out in compliance with bioethical standards. Animal housing, feeding, and care, as well as animal removal from the experiment, were carried out in accordance with: the requirements for the Care and Use of Experimental Animals of Kazan Federal University and of the experimental unit of the Z.I. Alimchueva commercial poultry farm (Medvedevsky District, Mari El, Russia). All animal management and experimental procedures for this study were approved by the Local Ethics Committee of KFU (Permit number: 22) and carried out in accordance with the Directive of the European Parliament and Council on the protection of animals used for scientific purposes dated September 22, 2010 (Directive 2010/63/UE on the protection of animals used of scientific purposes).
A total of 180 1-day-old chickens Cobb 500 were obtained from a Non-public joint stock company Mariskoe (Medvedevsky District, Mari El, Russia). Birds were neither vaccinated nor separated on a gender basis. For all aspects of the study, we involved both male and female birds at equal proportions. After weighing birds individually for equal weight distribution, an initial average body weight of 47.17 ± 3.13 g for all birds was recorded. The broilers were allocated to 12 battery cages and sorted into two primary groups (Control and Probiotic). Each group had six replicates, with each replicate (per cage) comprising 15 birds. The Control group (C group) was fed with a basal diet, whereas birds of the Probiotic group (P group) were fed the basal diet supplemented with 1 × 107 spores/g of B. subtilis GM5.
The chickens had free access to feed and water. At each diet switch, feeders were emptied, orts were weighed back and the feeders were filled with diets. The surrounding temperature of birds was maintained at 35–36 °C from days 1 to 5, 30–32 °C for the next 6 to 10 days, 26–28 °C from the day 11 to 20, and finally at 20–24 °C from day 21 until slaughter. The experiment lasted 42 days. Photoperiod program was set up according to the European welfare regulation 43/2007 (Council Directive 2007/43/EC, laying down minimum rules for the protection of chickens kept for meat production).
Birds were raised on a three-phase diet. Starter diets were offered to the broilers from days 0 to 10, grower diets from days 11 to 20, and finisher diets from days 21 to 42. The chemical composition of the feed rations (Algorithm Investments LLC) is shown in Supplementary Tables S1a, S1b, S1c.
Observations on the general condition of the herd, temperature, light, water, feed, litter condition and mortality were recorded twice a day. Room temperature and relative humidity were also recorded daily and adjusted accordingly to avoid the influence of stressful conditions on broiler chickens.
Growth Performance indicators
Body weight (BW) of broilers was measured on days 0, 10, 20, and 42. Average daily weight gain (ADWG) was calculated at 42 days of age. Feed intake (FI) was evaluated weekly, and subsequently re-estimated for a single bird. Feed conversion ratio (FCR) was obtained on the 42nd day of age, and the European productivity index (EPI) of broilers was calculated by the formula:
Sample collection, DNA extraction, and 16S rRNA gene sequencing
Since the feed rations of birds were altered on days 1, 10, and 42 based on their growth and physiological needs, six birds were randomly selected from each group for microbiome analysis on these days. They were euthanized with cervical dislocation followed by decapitation. Immediately after euthanasia, the abdominal cavity was opened, the ceca of each bird were incised, and the contents of both ceca were collected in a sterile 3 mL tube, frozen using liquid nitrogen, and transported to the laboratory on ice, then stored at − 80 °C until DNA extraction. Prior to total DNA isolation, the harvested cecal contents of two birds within each group were combined to obtain three replicates per treatment. In total, 18 samples for cecal contents were used for gut microbiota analysis. Chicken organs (spleen, liver, and heart) were collected and weighed immediately after euthanasia. The organ weight to whole body weight ratio was subsequently determined for each of the three organs.
Total genomic DNA was extracted from the 0.5 g cecal contents of each individual chicken using the commercially available QIAamp Fast DNA Stool Mini kit (QIAGEN, Germany) following the manufacturer's instructions. The quality and concentration of extracted DNA were measured using gel electrophoresis and Qubit 2.0 fluorometer (Life Technology, Carlsbad, USA). DNA was stored at − 20 °C until further processing.
PCR was carried out using Q5 Hot Start High-Fidelity 2X Master Mix (NEB, Great Britain) and universal primers 341F (5-CCT ACG GGN GGC WGC AG-3) and 805R (5-GAC TAC HVG GGT ATC TAA TCC-3) targeting V3–V4 variable regions of the bacterial 16S rRNA gene (Herlemann et al. 2011). The fragment distribution in the pooled library was evaluated using Agilent 2100 Bioanalyzer (Agilent Technologies, USA) and computed using Qubit 3.0 fluorimeter (Thermo Fisher Scientific, USA). The libraries containing 16S rRNA genes were sequenced by 2 × 300 bp paired-end sequencing on the MiSeq platform using MiSeq v3 Reagent Kit (Illumina, USA) at Joint KFU-Riken Laboratory, Kazan Federal University (Kazan, Russia).
OTU clustering and statistical analysis
The Illumina paired-end raw reads of each sample were quality proven using the FastQC v0.11.9 program. Metataxonomic analysis was performed using QIIME2 software, version 2020.2. Poor reads were filtered at the limit of Q20, chimeric sequences were removed by the USEARCH v.10.0 method. After quality filtration and eliminating chimeric sequences from raw reads, clean sequences were clustered into Operational Taxonomic Units (OTUs) with 97% similarity cut off. OTU picking steps were performed using pick_open_reference_otus.py script with the default UCLUST approach. The minimum specified OTU size required to keep an OTU was 5, the any OTU which failed to meet this criterion was removed from the further analysis. For the taxonomic classification of the reads, the RDP database v. 2.9 was used. The minimum confidence to record an assignment for rdp classification was 0.8.
After assigning the taxonomy, we used OTUs with a minimum relative abundance of 0.01% of the OTUs per sample. The resulting biom file was summarized at the different taxonomic levels using summarize_taxa.py. For data visualization and statistical analysis of diversity metrics, the computing medium R, version 3.6.3 was used. Graphs were computed with biom file data and plotted using the R packages phyloseq v. 1.32.0 and vegan v. 2.5–6. Alpha diversity was assessed using rarefaction plots, Shannon and Chao1 indices. PCoA plots for beta-diversity analysis were computed using unweighted UniFrac and Bray–Curtis distance matrices among samples.
Additional statistical processing of the results was performed in Graph Pad Prism, Graph Pad Software (LA Jolla, CA, USA) using one-way ANOVA and Tukey test for multiple pairwise comparisons of performance indicators. The results were presented as the mean ± SD, considering P value < 0.05 as significant. Data on relative organ mass-to-bodyweight of chickens were statistically analyzed using the Student t test.
Results
Overall Performance
The average bodyweight, daily weight gain, FI, and total feed conversion ratio were calculated at the indicated time points (1, 10, 20, and 42 days) for each of the two broiler groups (Table 1). The average body weight was higher (P < 0.05) in the probiotic group (P-group) than in the control group (C-group). After 10 days body weight gain (BWG) increased by 13.09% (P > 0.05) in the P-group in comparison with the C-group. FI was higher in the control group by 16.82% (P > 0.05). However, after 20 days, the BWG of the experimental group exceeded that of the control group by an average of 15.26% (P < 0.0001). In addition, feed intake was higher by 24.03% (P < 0.0001), respectively. On day 42, the increase in live weight of chickens of the experimental group exceeded the control by 12.97% (P < 0.0001), and the consumption of animal feed by 11.22% relative to the control (P < 0.0001). FCR in the probiotic group (days 1–42) was lower than in the control group by 1.44%. ADWG in the control and probiotic groups amounted to 49.71 g and 56.30 g (P < 0.05), respectively. The EPI in the control group was 244.43, as against 280.14 in the experimental group (P < 0.0001). Thus, the addition of B. subtilis GM5 spores to broiler feed leads to improved feed intake and digestibility, which is paramount in reducing the cost of the final product.
Table 1.
Effect of Bacillus subtilis GM5 supplementation on the growth performance in broiler chickens
| Parametrs | Day | Treatment |
P value P vs. C |
|
|---|---|---|---|---|
| C (mean ± SD) | P (mean ± SD) | |||
| Body weight (kg) | 10 | 0.275 ± 0.005 | 0.311 ± 0.006 | n.s |
| 20 | 0.603 ± 0.024 | 0.695 ± 0.039 | **** | |
| 42 | 2.135 ± 0.085 | 2.412 ± 0.106 | **** | |
| Feed intake (kg) | 10 | 0.333 ± 0.007 | 0.389 ± 0.007 | n.s |
| 20 | 1.186 ± 0.051 | 1.471 ± 0.088 | **** | |
| 42 | 4.349 ± 0.176 | 4.837 ± 0.216 | **** | |
| Feed conversion ratio | 1–42 | 2.08 | 2.05 | |
| Daily weight gain (g) | 42 | 49.71 ± 2.02 | 56.30 ± 2.52 | ** |
| European productivity index (g) | 42 | 244.43 ± 9.71 | 280.14 ± 12.25 | **** |
n.s. ** and **** correspond to not significant, significant (P < 0.05) and highly significant (P < 0.0001), respectively
The ratio of organ mass to body weight for the heart (P = 0.585), liver (P = 0.515), and spleen (P = 0.599), was statistically indifferent among birds in the control and experimental groups on day 42 (Table 2)
Table 2.
Relative organ to body weight of broilers at day 42
| Organs | C-group | P-group | P-value |
|---|---|---|---|
| Hearta | 6.408 ± 0.533 | 6.678 ± 0.580 | 0.585 |
| Livera | 31.206 ± 2.148 | 29.573 ± 3.337 | 0.515 |
| Spleena | 1.477 ± 0.366 | 1.280 ± 0.474 | 0.599 |
Bacterial community structure and diversity of cecal microbiota
DNA sequencing data analysis
Cecal samples were collected, and DNA was sequenced. 116,119 raw paired-end reads were obtained on average for each sample, and following assembly, 75,620 raw spliced tags remained. The trimmed and merged sequences were clustered into OTUs at 97% similarity using uclust. A total of 242 ± 26 and 360 ± 30 OTUs (day 1), 1517 ± 78 и 1602 ± 60 (day 10), as well as 1362 ± 228 and 1628 ± 51 (day 42) were identified for C- and P-groups, respectively (Supplementary Fig. 1). Data saturation was achieved and evaluation of the OTU richness is illustrated via Rarefaction curves.
Microbial abundance and diversity analysis
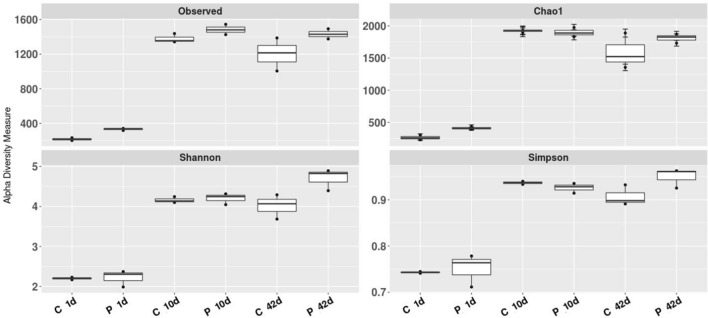
Chao1 indices were selected to identify community richness, and the Shannon index was used to identify community diversity. Shannon–Wiener’s Index of cecal bacterial communities in the C-group was lower (P < 0.05), relative to that of the P-group. Shannon indices of 3.16 ± 0.04 and 3.20 ± 0.30 (day 1), 6.01 ± 0.11 and 6.06 ± 0.19 (day 10), along with 5.72 ± 0.35 and 6.81 ± 0.40 (day 42) were recorded for the C- and P- groups, respectively (Fig. 1, Supplementary Table 4). The species diversity in the probiotic group on the 42nd day was significantly higher than in the control group (P < 0.05), based on Shannon’s diversity index. In the 1-day-old chicks, Chao1 Index considerably differed between the C-group and the P-group (P < 0.05), being 278.67 ± 35.92 and 415.00 ± 17.09, respectively. On days 10 and 42, the cecal composition richness (Chao1) was slightly but insignificantly greater in the probiotic group than in the control group (Fig. 1, Supplementary Table 4). Using Simpson’s index, a higher diversity was established in the P-group on both days 1 and 42 in comparison to the C-group (Supplementary Table 4).
Fig. 1.
Comparative differences in bacterial community diversity, richness, and cecal microfloral structure of the P-group and C-group based on Observed OTUs, Chao1, Shannon and Simpson indices. C 1d, C 10d and C 42d correspond to—control group birds on days 1, 10 and 42, respectively. P 1d, P 10d and P 42d refer to probiotic group birds on days 1, 10 and 42, accordingly
To assess the effect of probiotic treatment and growth stage on the gut microbiome, we calculated the unweighted UniFrac distance matrices. Principal coordinate analysis (PCoA) plots for unweighted UniFrac metrics were constructed to evaluate similarities between samples (Fig. 2). The coordinates of the PCoA plots explained 79.22% variation. As seen in Fig. 2, a significant clustering pattern is observed in the PCoA plots, presenting similarity in the microbiome of each growth stage (1-, 10- and 42-days of age). Bacterial communities formed comparatively a closer cluster on day 10, relative to the microbiota of the 1st and 42nd days. Significant differences in the structure of bacterial communities are confirmed by data on changes in the composition and structure of microbiota during its maturation. The intake of probiotics triggers a microbial shift, but not as significant as age-related changes (Fig. 2). Moreover, the most significant structural changes in the P-group microbiota were observed in broilers on days 1 and 42.
Fig. 2.
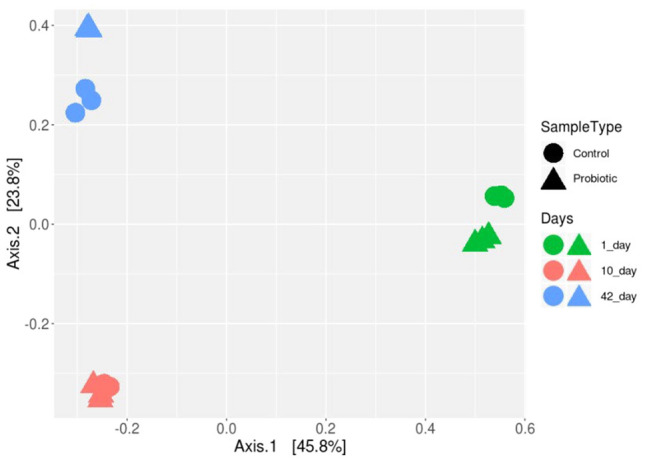
Pairwise comparison based on unweighted Unifrac distances between cecal microbial communities in broilers supplemented with probiotic (P-group) and broilers fed without probiotics (C-group), on days 1, 10 and 42 of growth. The Principal Component Analysis plot (PCoA) based on Bray–Curtis dissimilarities confirmed bacterial community differences centered on bird’s age. The unweighted UniFrac distance PCoA plots based on treatment did not unveil any significantly distinct clustering pattern between the C- and P-groups
Analysis of dominant bacterial taxa
From the cecal samples of groups, 9 phyla, 17 classes, 20 orders, 38 families, and 68 genera were identified (Supplementary Table 5). Firmicutes, Proteobacteria, and Bacteroidetes were the predominant phyla (Fig. 3a). However, depending on the age of the chickens, changes in the representation of different phyla were observed. For instance, on day 1 Firmicutes and Proteobacteria were prevalent, while in samples of days 10 and 42—were occupied by Firmicutes and Bacteroidetes (Fig. 3a). On day 1 the proportion of Firmicutes was 53.87 ± 0.39% and 57.61% ± 1.38% in C-group and P-group, respectively. On days 10 and 42, the proportion of Firmicutes were 56.01 ± 3.65% and 54.55 ± 7.12% in C-group, along with 54.74 ± 1.75% and 81.79 ± 9.42% in P-group, respectively. The abundance of Firmicutes in cecal communities of P-group significantly increased on day 42 in comparison to day 10 (P < 0.0001).
Fig. 3.
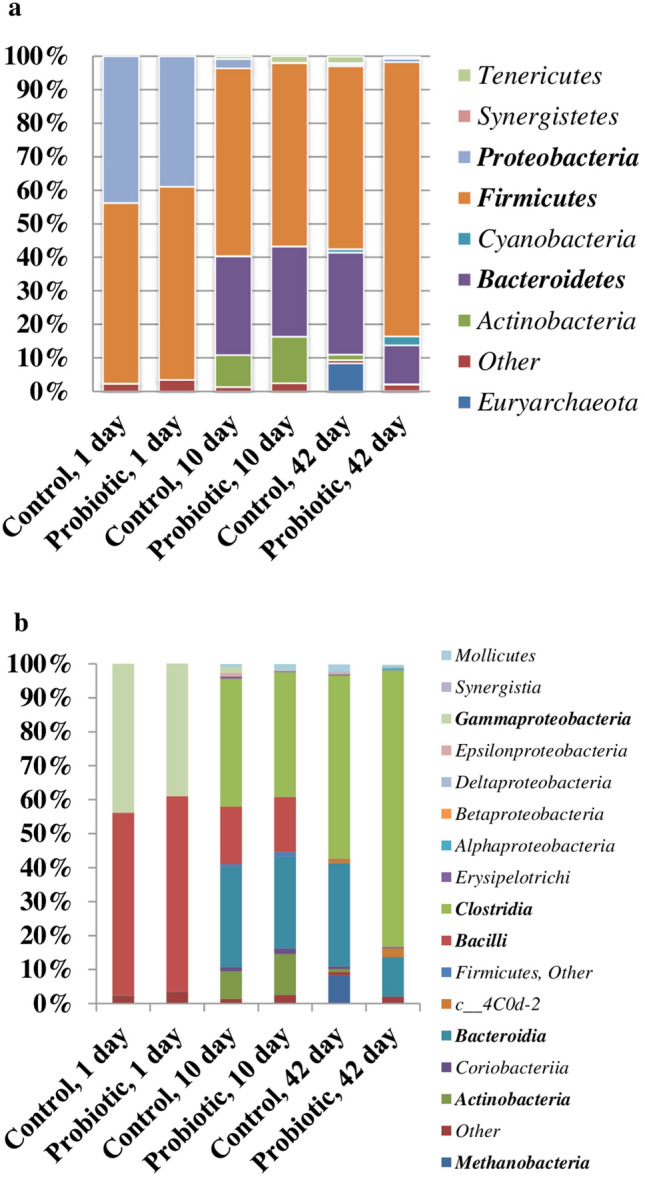
Relative abundances (%) of bacterial phyla (a), class (b) in the cecum of broiler birds in control and treatment (probiotic) groups. Birds were euthanized on days 1, 10 and 42 of age. Treatments represent birds fed with the spores of B. subtilis GM5
Proteobacteria were only significantly present (43.77 ± 0.42% and 38.93 ± 1.44% for C- and P-groups, respectively) in the cecum for 1-day old chickens in both groups. Subsequently, Proteobacteria in the cecal samples decreased to 2.79 ± 1.25% and 0.094 ± 0.07% on day 10, to 0.54 ± 0.18% and 1.01 ± 0.68% on day 42 in C- and P-groups, respectively. The proportion of Bacteroidetes also varied significantly depending on the age of the broilers. Bacteria of this group were practically absent in cecal samples of 1-day old chicks, but on the 10th day, their proportion increases to 29.50 ± 2.73% (C-group) and 26.94 ± 2.22% (P-group). On day 42, the proportion of Bacteroidetes virtually remained unchanged in the C-group (30.45 ± 2.39%), but significantly decreased in the group of chickens treated with probiotic (11.65 ± 6.32%). It was interesting to note that representatives of the phylum Actinobacteria were significantly detected only in samples of 10-day old chickens and their share amounted to 9.48 ± 0.3% and 13.79 ± 5.71% in C- and P-groups, respectively (Fig. 3a). In cecal samples of 1 day-old chicks, their share was less than 0.1%. In 42 day-old birds, the proportion of these bacteria was as low as 1.63 ± 0.52% and 0.09 ± 0.02% in C- and P-groups, respectively. The members of phyla Euryarchaeota, Cyanobacteria, Synergistetes, and Tenericutes represented minor communities of the gut microbiota (Supplementary Table 5).
At the class level, Firmicutes were primarily represented by the classes Bacilli and Clostridia (Fig. 3b, 4a), but their proportions reduced sharply. On day 1, the abundance of Bacilli was 53.45–54.23% (C-group) and 56.21–58.95% (P-group) but significantly decreased to 16.89 ± 2.02% and 16.23 ± 2.57% on day 10, respectively (P < 0.0001). Conversely, the abundance of Clostridia, significantly increased on day 10, relative to day 1 samples and amounted to 37.66 ± 1.66% in C-group (P < 0.0001) and 36.71 ± 1.71% in P-group (P < 0.0001). On day 42 the cecal microbiota was predominantly inhabited by Clostridia, which occupied more than 53% in C-group and 81% in P-group, respectively.
Fig. 4.
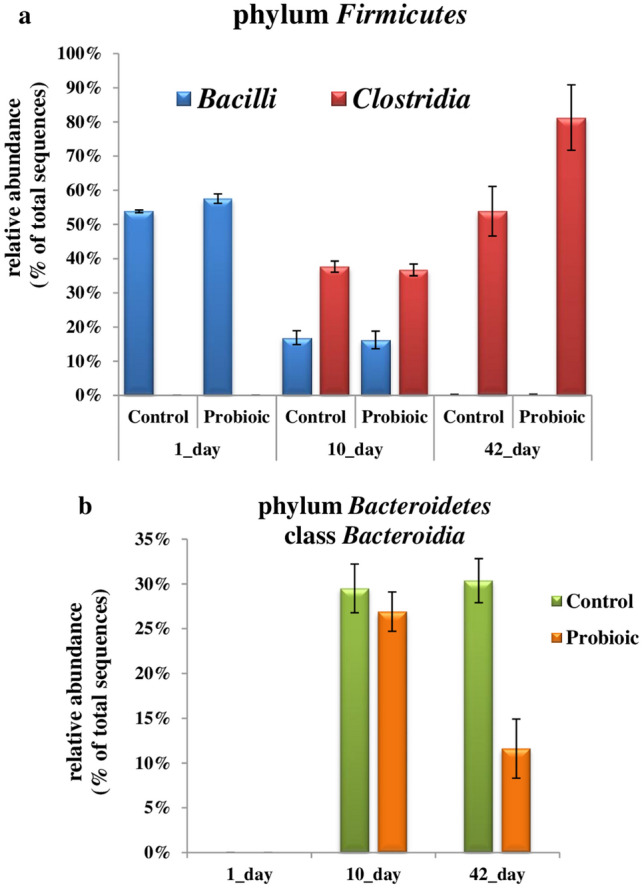
Relative abundance of different classes within phylum Firmicutes (a) and phylum Bacteroidetes (b) in the cecum of the control and probiotic groups of broilers on days 1, 10 and 42 of age
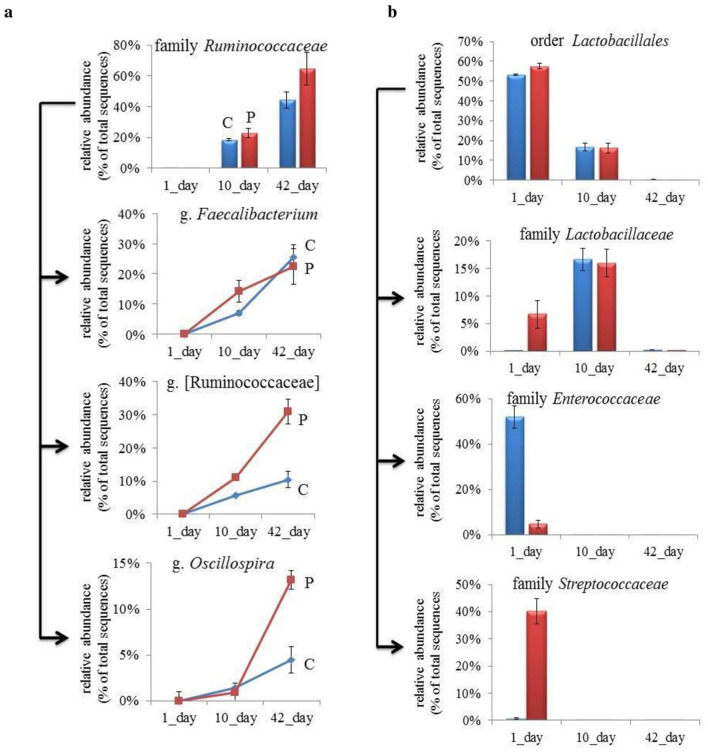
Within Firmicutes, the majority belonged to the Lachnospiraceae, Ruminococcaceae families (order Clostridiales), and Enterococcaceae, Lactobacillaceae, Streptococcaceae families (order Lactobacillales) (Fig. 5a, b). The relative proportions of these bacteria varied depending on age and availability of feed additives. In particular, the cecum of 1-day old chickens was first colonized by different representatives of Lactobacillales (53.25 ± 0.35% and 57.49 ± 1.39% in C- and P-groups, respectively). Different families of the Lactobacillales order showed dominance in the control and experimental groups: in the C-group, the Lactobacillales order was represented chiefly by Enterococcaceae (51.92 ± 0.50%) and Streptococcaceae (0.69 ± 0.22%). The P-group presented an inverted picture, in which samples were predominantly occupied by Streptococcaceae (40.10 ± 4.75%) and Enterococcaceae (4.76 ± 1.72%).
Fig. 5.
Relative abundance of different genera within f. Ruminococcaceae (a) and different families of the order Lactobacillales (b) in the control (C) group and probiotic (P) group on days 1, 10 and 42 of age
The share of members of the Lactobacillaceae family in 1-day-old chickens varied considerably in the two groups and amounted to 0.06 ± 0.02% and 6.7 ± 2.49% in the control and probiotic group, respectively. It could be noted that in cecal samples of 10 day-old birds, the order of Lactobacillales is represented almost exclusively by Lactobacillaceae, the proportion of which were recorded at 16.64 ± 1.96% and 15.99 ± 2.52% in C- and P-groups, respectively. However, by the 42nd day, the abundance of Lactobacillaceae and other bacteria of this order had significantly decreased in both groups of the broiler birds (P < 0.0001). A decrease in the share of Lactobacillales on the 10th day correlated with an increase in the presence of Lachnospiraceae and Ruminococcaceae families of Clostridiales (C 37.65 ± 1.69%, P 36.68 ± 1.70%). The proportion of Lachnospiraceae in the control group (12.16 ± 0.45%) was higher than that of the experimental group (6.35 ± 0.52%) on the 10th day. By day 42, the proportion of these bacteria had decreased to 2.38 ± 0.4% (C) and 0.15 ± 0.002% (P).
On day 10 the proportion of Ruminococcaceae was 18.47 ± 0.74% in the C-group and 22.54 ± 3.07% in the P-group but significantly increased to 44.30 ± 5.45% and 64.67 ± 10.67% on day 42, respectively. Thus, the use of B. subtilis GM5 as probiotics led to a significant increase (P < 0.0001) in the number of Ruminococcaceae during the development of the cecal bacterial community.
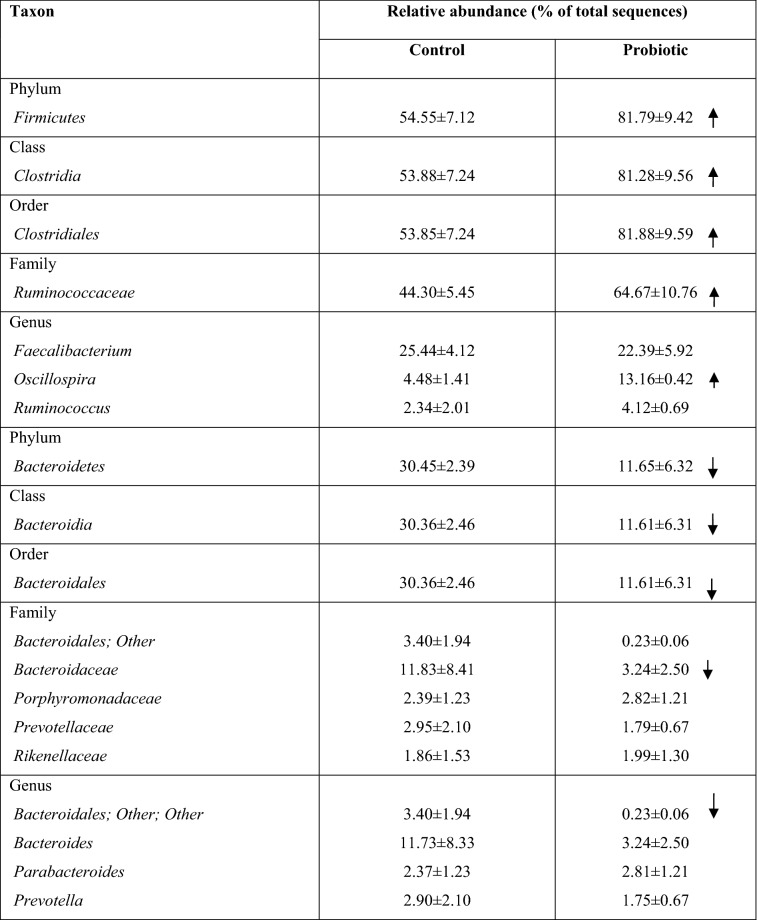
At the genus level, different representatives exhibited dominance depending on the age of birds (Fig. 5). On day 1, Firmicutes were represented by Enterococcus (46.89 ± 0.38%) in the C-group and Streptococcus (38.70 ± 5.08%) in the P-group. On the 10th day, the cecal microbiota in the C- and P-groups was occupied mainly by Lactobacillus (16.37 ± 1.85% and 15.33 ± 2.55%) and Faecalibacterium (7.07 ± 0.8% and 14.16 ± 3.64%). Moreover, the proportion of Faecalibacterium in the probiotic group was significantly higher than that of the control group (P < 0.0001). On day 42, the two groups were highly populated with Faecalibacterium (25.44 ± 4.12% and 22.39 ± 5.92% for C-group and P-group, respectively), the proportion of which was significantly higher than on the 10th day (P < 0.01) (Table 3). In addition, Firmicutes were chiefly represented on day 42 by unclassified members Ruminococcaceae and Oscillospira, the relative abundances of which were two and three times higher in the P- and C-groups, respectively (P < 0.0001) (Fig. 5, Table 3).
Table 3.
The relative abundance of the main bacterial taxa in the cecum of broiler chickens on day 42 and the effect of the probiotic B. subtilis GM5
Mean percentages and standard error (S.E.) of bacterial abundance observed from 18 samples
Arrows indicate an increase (
 ) or decrease (
) or decrease (
 ) in the relative abundance (% of total sequence)
) in the relative abundance (% of total sequence)
The phylum Proteobacteria was predominantly represented by the class Gammaproteobacteria, representation of which covered 43.77 ± 0.42% and 38.93 ± 1.44% in C- and P-groups, respectively (P < 0.01) on day 1. However, on day 10 Gammaproteobacteria decreased significantly to 1.7 ± 0.61% in C-group and 0.05 ± 0.02% in P-group (P < 0.0001). Within the Enterobacteriaceae family, the majority corresponded to the genera Escherichia (C-group = 18.42 ± 2.48 and P-group = 37.77 ± 1.56%) and Klebsiella (C-group = 13.88 ± 2.94% and P-group = 0.04 ± 0.0%) (Supplementary Table 5).
The abundance of the dominant class Bacteroidia was 29.51 ± 2.73% (C-group) and 26.94 ± 2.22% (P-group) in the day 10 sample, but in 42-day-old chickens, they occupied 30.45 ± 2.39% in the control group but decreased to 11.65 ± 6.32% in the probiotic group (Fig. 4b). The principal genera were Barnesiella and Bacteroides (Table 3).
On day 10, the principal family and genus of Actinobacteria were Bifidobacteriaceae and Bifidobacterium, the abundance of which were, respectively, higher (P < 0.05) in the P-group (12.07 ± 5.16% and 10.14 ± 4.20%) than in the C-group (8.17 ± 0.49% and 7.40 ± 0.41%). However, their representation significantly decreased by day 42.
An important parameter is a ratio between the two dominant phyla, Firmicutes and Bacteroides (F/B ratio), the total share of which amounts to 81–85% in the cecum on the 10th day and 85–93% on the 42nd day. It is interesting to note that the use of the probiotic significantly increased the F/B ratio, especially in 42-day-old birds. To be precise, the F/B ratio in the control group was approximately at the same level and amounted to 1.89 (10th day) and 1.79 (42nd day). On the other hand, the ratio value of the experimental group increased to 2.03 and 7.02 on days 10 and 42, respectively. The higher number of Firmicutes in the experimental group directly correlates with the higher weight gain of broiler birds in the experimental group on the 42nd day. Spearman's correlation analysis showed a positive correlation between the F/B ratio and chickens’ body weight (Spearman ρ = 1.0, P < 0.05).
Discussion
The results of the present study demonstrate that supplementation with the spores of B. subtilis GM5 (1.0 × 107 CFU/g) as probiotic in broiler diets can promote broiler weight gain and daily weight gain while lowering FCR. The average body weight of the P-group chickens was higher than the mean body weight of the C-group by 15.26% (P < 0.0001) on day 20 and 12.97% (P < 0.0001) on day 42. FCR of broilers from the 1st to the 42nd day was 1.44% lower in the P-group than in the control. It has been shown that dietary supplementation with B. subtilis CGMCC 1.1086, B. subtilis UBT-MO2 and three other B. subtilis strains effectively improves the growth performance and FCR of broilers via the beneficial modulation of cecal microbiota (Amerah et al. 2013; Zhang et al. 2013; Li et al. 2016). Some probiotics exert a minimal effect on the growth rates of broiler chickens (Mountzouris et al. 2007; Lee et al. 2010; Jerzsele et al. 2012). These discrepancies in results could be tied to differences in strains used as probiotics, the dosage of administration, preparation methods, poultry age, dietary composition and hygiene (Lee et al. 2010; Zhang et al. 2012; Li et al. 2019). The formation of the intestinal microbiota of broiler chickens depends on several factors and undergo regular age-related changes (Xu et al. 2016; Kers et al. 2018; Ngunjiri et al. 2019). Noteworthy differences in the composition of the cecal microbiota were found in chickens raised under wild conditions and those bred on farms (Pandit et al. 2018; Kubasova et al. 2019; Rychlik 2020).
Microbes inhabiting the intestines of a day-old chick are classified as variable microbiota, thus the colonization and composition of the original intestinal microbiota of hatched chickens are expected to vary significantly among individual birds from different incubators (Pedroso et al. 2005; Kers et al. 2018). This explains the divergence in results obtained by various researchers. For example, Ballou et al. (2016) showed that the dominant group in the cecum on the first day are Enterobacteriaceae (85%) (phylum Proteobacteria). In contrast, another study reported a relative dominance of Pelotomaculum (o. Clostridiales) and Enterococcus (o. Lactobacillales) in the cecum (Pedroso et al., 2016). Upon arrival, chickens are exposed to a more diversified microbial environment at the farm, consisting primarily of bacteria from litter, feed, and water. Succession emerges so rapidly that the microbiota of the intestines begins to differentiate from the third day of age in poultry birds (Lu et al. 2003). The cecal microbiota of a 3-day old chick is predominantly populated with Firmicutes, in particular members of the Ruminococcaceae family, as well as other representatives of the order Clostridiales. The prevalence of these groups of bacteria persists in birds even until the 28th day of age (Caporaso et al. 2012). It has been reported that the GI of a 7-day old bird is primarily inhabited by Flavonifractor, Pseudoflavonifractor, and Lachnospiraceae (o. Clostridiales), but with age (from 7 to 42 days), the diversity of the cecal microbiota steadily increases (Oakley et al. 2014a, b). In another study, it was shown that the displacement of Enterobacteriaceae bacteria with Clostridiales occurs on day 14 (Wise and Siragusa, 2007). Similar results were found in other investigations, confirming that the cecum is first colonized by Enterobacteriaceae, Lactobacillus, and Bifidobacterium, which are later replaced by Clostridiales (Zhu and Joerger 2003).
Our studies revealed that the cecum of broilers was initially colonized by gram-positive Firmicutes and gram-negative Proteobacteria, the proportion of which was approximately equal in both control and experimental birds. However, these phyla were represented by diverse dominant families in fowls, depending on their diet. To be precise, Firmicutes were represented mainly by Enterococcaceae, in the cecum of control chickens and Streptococcaceae in experimental chickens. The structure of representatives of Enterobacteriaceae likewise varied. The enterobacteria Escherichia and Klebsiella were present in the control birds, while the absence of Klebsiella was noted in chickens fed with the probiotic. Thus, the use of a probiotic led to changes in microbiota even at the very early stages of cecal colonization, causing a decrease in the representation of the bacterial groups Enterococcaceae and Klebsiella, among which many pathogenic strains are classified.
On the 10th day, the chickens showed significant changes in the cecum microbiota. These changes occurred primarily due to the maturation of the microbiota and correlated with probiotic use (Fig. 2). In both groups, the previously dominant Bacilli group is partially replaced by Clostridia. Subsequently, the emergence of Bacteroidia and Actinobacteria (fam. Bifidobacteriaceae) in the community is observed, as the share of Enterobacteriales significantly decreases. The addition of a probiotic leads to a more significant decrease in the proportion of Enterobacteriales, which is a positive factor. But in general, it shows that on day 10, beta-diversity of the cecal microbiota in experimental and control chickens does not differ much in terms of bacterial composition. Data from a previous study suggested that gut the microbiome is differentially affected by age than treatment (Ballou et al. 2016). The relatively high representation of groups such as Bifidobacteriaceae and Lactobacillaceae in the cecum of chickens in the early stages of development seems to be a natural stage in gut microbiota development. Thus, as the cecal microbiota matures, Enterobacteriales is gradually replaced by Bacteroidales and Bifidobacteriales.
On day 42, besides significant age-related changes, substantial differences are revealed in the microbiota structure of the control and experimental groups (Fig. 2, 4, 5a). In both groups, clostridial members almost completely ousted Lactobacilli, which are usually present in early life. Moreover, the proportion of Clostridiales in experimental broilers (81.79%) was significantly higher as compared to the control (54.55%) (Table 3, Fig. 4), while the proportion of Bacteroidales was 2.6 times lower in experimental chickens than in the control.
The bacteria of the two dominant phyla, Bacteroidetes and Firmicutes are known to play a vital role in the digestion of nutrients. The genomes of Bacteroides contain many genes involved in the metabolism and degradation of complex polysaccharides and mono sugars. These genes are likewise known to actively produce organic acids, as well as encode proteins and enzymes that play a central role during interactions with their hosts (Magnusdottir et al. 2017; Medvecky et al. 2018). Many representatives of Bacteroidetes and Firmicutes are capable of synthesizing short-chain fatty acids, especially butyrate (Anand et al. 2016; Medvecky et al. 2018). Several members of the family Ruminococcaceae can digest the cellulose in feed and produce short-chain fatty acids, as well as play essential roles in the digestions of lipids (Li et al. 2019; Medvecky et al. 2018).
It was shown that increased Firmicutes/Bacteroidetes (F/B) ratios were associated with growth promotion in chickens (Mancabelli et al. 2016; Salaheen et al. 2017). A high F/B ratio in the cecum leads to the active fermentation of volatile fatty acids, contributing to the deposition of fat (Mancabelli et al. 2016). In this study, the addition of B. subtilis GM5 facilitated bird growth and modulated the microbiota and, in particular, increased the F/B ratio in the experimental group relative to the control. The data obtained indicate that an increase in the level of Firmicutes, and Clostridiales in broiler ceca may be one of the many factors contributing to the enhanced growth of broiler chickens.
The Firmicutes phylum includes a wide range of beneficial bacteria, such as Ruminococcaceae, Lactobacillaceae, Lachnospiraceae, and Streptococcaceae. Probiotic strains of Bacillus are aerobic bacteria that consume large amounts of free oxygen, as well as secrete various metabolites when growing in the intestinal tract. As a result, they inhibit the growth of most pathogenic bacteria (Escherichia, Salmonella, etc.) and enhance the growth of beneficial anaerobic bacteria, such as Lactobacillus and Bifidobacterium (Wang et al. 2006; Gao et al. 2017). Being a transient member of the intestinal microbiota B. subtilis does not colonize the intestines. On the contrary, they can increase the relative prevalence of beneficial microbiota in the cecum and, apparently, contribute to faster maturation of the gut microbiota and increase its diversity.
Conclusion
The results of this study demonstrate that supplementing the diet of broilers with spores of B. subtilis strain GM5 improves productivity by increasing the weight gain of birds and reducing feed conversion, as well as increases diversity and the relative abundance of beneficial microbiota in the cecum. We conclude that the addition of the probiotic from the first days of life can regulate and stabilize the microbiota of the digestive tract of chickens, which is essential for growth and development.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The culturing of Bacillus subtilis GM5 and the obtainment of spores were supported by the research grant of Kazan Federal University. All other experiments were supported by a grant from the Russian Science Foundation (No. 16-16-04062).
Author contributions
MR AM designed the research and supervised all research; GH, MT, NG, DP, YA carried out the experiments and analyzed the data and drafted; DP, GH prepared figures; GH, AM, YA wrote the manuscript; MS, AM, ES partook in the revision of the manuscript. All authors have read and approved the final version of the manuscript.
Availability of data
All datasets generated for this study have been included in the article/Supplementary Material. Sequence data has been uploaded to the European Nucleotide Archive and can be accessed from the website https://www.ebi.ac.uk/ena using the accession number PRJEB37602.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Amerah AM, Quiles A, Medel P, Sánchez J, Lehtinen MJ, Gracia MI. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim Feed Sci Technol. 2013;180:1–4. doi: 10.1016/j.anifeedsci.2013.01.002. [DOI] [Google Scholar]
- Anand S, Kaur H, Mande SS. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front Microbiol. 2016;7:1945. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G, Croubels S, Pasmans F, Ducatelle R, Eeckhaut V, Devreese M, et al. Fumonisins affect the intestinal microbial homeostasis in broiler chickens, predisposing to necrotic enteritis. Vet Res. 2015;46:98. doi: 10.1186/s13567-015-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K, Huang Q, Zhang J, He J, Zhang L, Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Ballou AL, Ali RA, Mendoza MA, Ellis JC, Hassan HM, Croom WJ, et al. Development of the chick microbiome: how early exposure influences future microbial diversity. Front Vet Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112:5649–5654. doi: 10.1073/pnas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Lee TK, Sul WJ. Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens—a review. Asian-Australas J Anim Sci. 2015;28:1217–1225. doi: 10.5713/ajas.15.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clench MH, Mathias JR. Motility responses to fasting in the gastrointestinal tract of three avian species. Condor. 1995;97:1041–1047. doi: 10.2307/1369542. [DOI] [Google Scholar]
- Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F, et al. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R, Eeckhaut V, Haesebrouck F, Van Immerseel F. A review on prebiotics and probiotics for the control of dysbiosis: present status and future perspectives. Animal. 2015;9:43–48. doi: 10.1017/S1751731114002584. [DOI] [PubMed] [Google Scholar]
- Gadde U, Oh ST, Lee YS, Davis E, Zimmerma N, Rehberger T, Lillehoj HS. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Gao Z, Wu H, Shi L, Zhang X, Sheng R, Yin F, et al. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens. Anim Nutr. 2017;3:109–113. doi: 10.1016/j.aninu.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadieva GF, Lutfullin MT, Pudova DS, Akosah YA, Shagimardanova EI, Mardanova AM, et al. Data on the genome analysis of the probiotic strain Bacillus subtilis GM5. Data Brief. 2019;23:103643. doi: 10.1016/j.dib.2018.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlemann DP, Labrenz M, Jurgens K, Bertilsson S, Waniek JJ, Andersson AF. Transitions in bacterial communities along the 2000 km salinitygradient of the Baltic Sea. ISME J. 2011;5:1571–1579. doi: 10.1038/ismej.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Cheng Y, Li Y, Li X, Zhou Z, Shi D, et al. Preliminary study on the effect of Bacillus amyloliquefaciens TL on cecal bacterial community structure of broiler chickens. Biomed Res Int. 2019;2019:5431354. doi: 10.1155/2019/5431354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Zhang Y, Xiao K, Jiang F, Wang H, Tang D, et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6:211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzsele A, Szeker K, Csizinszky R, Gere E, Jakab C, Mallo JJ. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Jozefiak D, Rutkowski A, Martin SA. Carbohydrate fermentation in the avian ceca: a review. Anim Feed Sci Technol. 2004;133:1–4. doi: 10.1016/j.anifeedsci.2003.09.007. [DOI] [Google Scholar]
- Kers JG, Velkers FC, Fischer EAJ, Hermes GDA, Stegeman JA, Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadieva GF, Lutfullin MT, Mochalova NK, Lenina OA, Sharipova MR, Mardanova AM. New Bacillus subtilis strains as promising probiotics. Mikrobiology. 2018;87:463–471. doi: 10.1134/S0026261718040112. [DOI] [Google Scholar]
- Kubasova T, Kollarcikova M, Crhanova M, Karasova D, Cejkova D, Sebkova A, et al. Contact with adult hen affects development of cecal microbiota in newly hatched chicks. PLoS ONE. 2019;14:3. doi: 10.1371/journal.pone.0212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kyung D, Lillehoj HS, Jang SI, Lee SH. Immune modulation by Bacillus subtilis-based direct-fed microbials in commercial broiler chickens. Anim Feed Sci Technol. 2015;200:76–85. doi: 10.1016/j.anifeedsci.2014.12.006. [DOI] [Google Scholar]
- Lee K, Lillehoj HS, Siragusa GR. Direct-fed microbials and their impact on the intestinal microflora and immune system of chickens. J Poult Sci. 2010;47:106–114. doi: 10.2141/jpsa.009096. [DOI] [Google Scholar]
- Li C, Wang J, Zhang H, Wu S, Hui Q, et al. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu Q, Huang Z, Lv L, Liu X, Yin C, et al. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. Appl Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Lu J, Idris U, Harmon B, Maurer JJ, Lee MD, Hofacre C. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusdottir S, Heinken A, Kutt L, Ravcheev DA, Bauer E, Noronha A, et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat Biotechnol. 2017;35:81–89. doi: 10.1038/nbt.3703. [DOI] [PubMed] [Google Scholar]
- Mancabelli L, Ferrario C, Milani C, Mangifesta M, Turroni F, Duranti S, et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Mardanova AM, Hadieva GF, Lutfullin MT, Khilyas IV, Minnullina LF, Gilyazeva AG, et al. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agric Sci. 2017;8:1–20. doi: 10.4236/as.2017.81001. [DOI] [Google Scholar]
- Medvecky M, Cejkova D, Polansky O, Karasova D, Kubasova T, Cizek A, et al. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken cecum in pure cultures. BMC Genom. 2018;19:561. doi: 10.1186/s12864-018-4959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountzouris KC, Tsitrsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Musa BB, Duan Y, Khawar H, Sun Q, Ren Z, Elsiddig Mohamed MA, et al. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J Anim Physiol Anim Nutr (Berl) 2019;103:1039–1049. doi: 10.1111/jpn.13082. [DOI] [PubMed] [Google Scholar]
- Neijat M, Shirley R, Kiarie E. Performance and apparent retention of nutrients in Shaver White pullets and laying hens in response to dietary supplementation of graded levels of a single strain Bacillus probiotic. Poult Sci. 2018;97:346. doi: 10.3382/ps/pez080. [DOI] [PubMed] [Google Scholar]
- Neijat M, Shirley RB, Welsher A, Barton J, Thiery P, Kiarie E. Growth performance, apparent retention of nutrient and excreta dry matter content in Shaver White pullets (5–16 week of age) in response to dietary supplementation of graded levels of a single strain Bacillus subtilis probiotic. Poult Sci. 2019;98:3777–3786. doi: 10.3382/ps/pez080. [DOI] [PubMed] [Google Scholar]
- Ngunjiri JM, Taylor KJM, Abundo MC, Jang H, Elaish M, Mahesh KC, et al. Farm stage, bird age, and body site dominantly affect the quantity, taxonomic composition, and dynamics of respiratory and gut microbiota of commercial layer chickens. Appl Environ Microbiol. 2019;85:9. doi: 10.1128/AEM.03137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BB, Buhr R, Ritz CW, Kiepper BH, Berrang ME, Seal BS, et al. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet Res. 2014;360:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. 2014;360:2. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Pandit RJ, Hinsu AT, Patel NV, Koringa PG, Jakhesara SJ, Thakkar JR, et al. Microbial diversity and community composition of cecal microbiota in commercial and indigenous indian chickens determined using 16s rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso AA, Batal AB, Lee MD. Effect of in ovo administration of an adult-derived microbiota on establishment of the intestinal microbiome in chickens. Am J Vet Res. 2016;77:514–526. doi: 10.2460/ajvr.77.5.514. [DOI] [PubMed] [Google Scholar]
- Pedroso AA, Menten JFM, Lambais MR. The structure of bacterial community in the intestines of newly hatched chicks. J Appl Poult Res. 2005;14:232–237. doi: 10.1093/japr/14.2.232. [DOI] [Google Scholar]
- Ranjitkar S, Lawley B, Tannock G, Engberg RM. Bacterial succession in the broiler gastrointestinal tract. Appl Environ Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhayat L, Jacquier V, Brinch KS, Nielsen P, Nelson A, Geraert PA, Devillard E. Bacillus subtilis strain specificity affects performance improvement in broilers. Poult Sci. 2017;96:2274–2280. doi: 10.3382/ps/pex018. [DOI] [PubMed] [Google Scholar]
- Richards P, Fothergill J, Bernardeau M, Wigley P. Development of the caecal microbiota in three broiler breeds. Front Vet Sci. 2019;6:201. doi: 10.3389/fvets.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals (Basel) 2020;10:1. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaheen S, Kim SW, Haley BJ, Van Kessel JAS, Biswas D. Alternative growth promoters modulate broiler gut microbiome and enhance body weight gain. Front Microbiol. 2017;8:2088. doi: 10.3389/fmicb.2017.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schokker D, Veninga G, Vastenhouw SA, Bossers A, Bree FM, Kaal-Lansbergen LM, et al. Early life microbial colonization of the gut and intestinal development differ between genetically divergent broiler lines. BMC Genom. 2015;16:418. doi: 10.1186/s12864-015-1646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Kumar S, Oakley B, Kim WK. Chicken gut microbiota: importance and detection technology. Front Vet Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmuzova K, Kubasova T, Davidova-Gerzova L, Sisak F, Havlickova H, Sebkova A, et al. Composition of gut microbiota influences resistance of newly hatched chickens to Salmonella enteritidis infection. Front Microbiol. 2016;7:957. doi: 10.3389/fmicb.2016.00957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I. Influence of Salmonella enterica serovar enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res. 2013;9:140. doi: 10.1186/1746-6148-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–135. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Wealleans AL, Walsh MC, Romero LF, Ravindran V. Comparative effects of two multi-enzyme combinations and a Bacillus probiotic on growth performance, digestibility of energy and nutrients, disappearance of non-starch polysaccharides, and gut microflora in broiler chickens. Poult Sci. 2017;96:4287–4297. doi: 10.3382/ps/pex226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise MG, Siragusa GR. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J Appl Microbiol. 2007;102:1138–1149. doi: 10.1111/j.1365-2672.2006.03153.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yang H, Zhang L, Su Y, Shi D, Xiao H, et al. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016;16:259. doi: 10.1186/s12866-016-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZF, Cho JH, Kim IH. Effects of Bacillus subtilis, UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livestock Sci. 2013;155:343–347. doi: 10.1016/j.livsci.2013.05.021. [DOI] [Google Scholar]
- Zhang ZF, Zhou TX, Ao X, Kim IH. Effects of ß-glucan and Bacillus subtilis, on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize-soybean meal based diets. Livest Sci. 2012;150:419–424. doi: 10.1016/j.livsci.2012.10.003. [DOI] [Google Scholar]
- Zhao L, Wang G, Siegel P, He C, Wang H, Zhao W, et al. Quantitative genetic background of the host influences gut microbiomes in chickens. Sci Rep. 2013;3:1163. doi: 10.1038/srep01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XY, Joerger RD. Composition of microbiota in content and mucus from caeca of broiler chickens as measured by fluorescent in situ hybridization with group-specific, 16S rRNA-targeted oligonucleotide probes. Poult Sci. 2003;82:1242–1249. doi: 10.1093/ps/82.8.1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study have been included in the article/Supplementary Material. Sequence data has been uploaded to the European Nucleotide Archive and can be accessed from the website https://www.ebi.ac.uk/ena using the accession number PRJEB37602.