Abstract
Owing to the lack of longitudinal studies in Latin American countries, we aimed to evaluate back pain and its risk factors in a 3-year longitudinal study of Brazilian adolescents. We analysed data of 525 adolescents (aged 11–16 years) attending primary school (fifth to eighth grade) in Brazil. The students were administered the self-reported Back Pain and Body Posture Evaluation Instrument (BackPEI) questionnaire in 2011 and at a follow-up evaluation that was conducted 3 years later (2014). Back pain was the outcome variable; the exposure variables included exercise, behavioural, hereditary and postural factors. Generalized estimating equations were used to perform a Poisson regression model with robust variance to evaluate the risk factors for back pain. The prevalence of back pain at baseline was 56% (n = 294); this increased significantly at the 3-year follow-up evaluation to 65.9% (n = 346). The frequency of experiencing back pain also significantly increased after 3 years in both boys (p = 0.002) and girls (p = 0.001). The prevalence of back pain increased significantly in adolescents up to the age of 13 years, stabilized in those aged 14 years and older and was higher among girls. A family history of back pain (in the parents), watching television for lengthy periods and carrying a backpack asymmetrically were predictors for back pain.
Keywords: Back pain, prospective, habits, posture, students, adolescent, epidemiology
Introduction
Back pain (BP) is a significant public health problem.1–5 Recent studies6,7 suggested that the prevalence of BP has been increasing in adolescents; this can cause a range of disabilities that may persist into adulthood.8–10 Therefore, the causes of BP must be investigated with a focus on adolescents.11 Although several recent studies examined the prevalence and risk factors of BP in adolescents,12–20 most had a cross-sectional design; thus, no causality could be established.
To date, few longitudinal studies have been performed on BP in adolescents and, to the best of our knowledge, all relevant published studies were performed in developed countries in Europe, North America and Asia.6,21–26 As pain during adolescence is an important predictor of pain in adulthood,27 an adequate assessment in children and adolescents is fundamental to help researchers and health professionals better understand BP and the associated risk factors and consequently improve health promotion programmes and interventions. Due to the lack of longitudinal studies in emerging countries, we aimed to evaluate BP and its risk factors by conducting a 3-year longitudinal study in Brazilian adolescents. This study, to the best of our knowledge, is the first to focus on Latin American adolescents, applying a longitudinal study protocol.
Methods
Study population
This study was performed in Teutônia, Rio Grande do Sul, Brazil, in 2011. According to the census,28 this municipality had approximately 32,000 inhabitants, a Human Development Index (HDI-2010) of 0.747 (HDI for Brazil at the same time, 0.699) and a total of 1720 fifth- to eighth-grade school children from 11 schools (9 public (n = 1575) and 2 private (n = 145) schools).
We estimated the sample size based on a finite proportion equation (N = 1720), assuming a 95% confidence interval (CI; z = 1.96), a proportion of 0.5 and an error of 4%, resulting in a value of 445 individuals. Predicting eventual losses, we invited 736 randomly selected students, who were selected based on the following steps: (a) research staff visits to all primary schools in the city (n = 11) to obtain authorization from the schools, (b) invitation of the students in the classrooms and requests for authorization to participate in the research to their parents or guardians and (c) return to school on a scheduled date to evaluate whose guardians signed the written consent form to participate in the study.
We included 726 primary students (fifth to eighth grade), aged 11–16 years, whose guardians were willing to let them participate in the study. Each participant was invited to participate in the follow-up study conducted 3 years later (2014). We excluded students who missed one of the baseline and follow-up examinations and those who were pregnant during the study period. Among baseline students, 201 did not participate in the follow-up assessment for the following reasons: not present on the day of the assessment, changed schools or dropped out of school; thus, they were excluded from the analysis.
The present study was performed in accordance with the Helsinki Declaration and was approved by the Ethics Research Committee from the Federal University of Rio Grande do Sul (number 19832).
Study design
The study was part of the ‘Brazilian longitudinal study on back pain and posture from adolescents’.29 An epidemiological exploratory and longitudinal study was conducted between October 2011 and October 2014.
Answers were obtained from students at two time points (2011 and 2014), using the Back Pain and Body Posture Evaluation Instrument (BackPEI), a self-administered questionnaire with confirmed validity and reproducibility.30 The questionnaire addresses the following issues: BP in the last 3 months (occurrence and frequency), heredity (BP in parents), behaviour (reading/studying in bed, time per day spent watching television/using a computer and sleep duration), exercise (physical exercise, weekly frequency of exercise and competitive exercise) and postural factors (sleeping posture; sitting posture to write, to use a computer and to talk; and the way of carrying school supplies).
The following questions evaluated the outcome of BP: (a) occurrence: ‘Have you felt back pain (or have you been in pain) in the last 3 months?’ (b) frequency: ‘How often do you feel (or felt) back pain?’ (c) impact on life activities: ‘Does the pain prevent (or has it prevented) you from performing daily life activities, such as playing, studying, practicing sports, etc’ and (d) intensity, evaluated from a visual analogue scale: ‘On a scale from 0 to 10 cm, please identify the intensity of your back pain for the last 3 months’.30
Questions related to posture such as sitting, lifting an object and carrying a backpack comprise figures showing subjects performing these activities, with a specific version for each sex. These diagrams facilitate the identification of the content of each question and the respondent’s interpretation of the question; consequently, they lead to a more representative response. Each question had five or six response options, including ‘another way/I don’t know’. Only one response option was considered as the correct way of performing an activity; the remaining alternatives were grouped as ‘incorrect’ in the statistical analysis.30
The BackPEI questionnaire was handed to each student in their classroom; how the questionnaire should be answered was explained collectively. The questionnaire contained personal information, such as name, name of father and mother and an identifier number from each student. These answers permitted us to identify all students even if they changed class or school.
Statistical analysis
The Statistical Package for the Social Sciences version 20.0 was used for all statistical analyses. Data were analysed using descriptive and inferential statistics. The chi-square test was used to compare the results between sexes for the baseline (2011) and follow-up (2014) evaluations. Using the McNemar test, a nonparametric test for related sample pairs, we compared (a) BP prevalence independent of age between baseline (2011) and follow-up (2014), (b) BP frequency between baseline (2011) and follow-up (2014) and (c) impact of day life activities.
We also performed the paired t test in order to compare the BP intensity between baseline (2011) and follow-up (2014) evaluations and the independent t test to compare BP intensity between sexes.
Generalized estimating equations (GEEs) were used to perform a Poisson regression model with robust variance for longitudinal data analysis.31,32 GEEs were used with an exchangeable correlation structure. The GEE methodology was indicated for this study as there were repeated measures in the between-subject and within-subject (2011 × 2014 evaluations) variables and because our data were binary.31,33 BP was the outcome; the exposure variables included exercise, behaviour, heredity and postural factors. Exposure variables with a significance level of p < 0.10 in the bivariate analysis were included in the multivariate regression model.34 We also adjusted the multivariate model for sex and age. We used relative risk (RR) with their respective 95% CIs as measures of effect (α = 0.05).
Results
Of the adolescents who participated in the baseline study (n = 726), 525 (73.2%) were revaluated after 3 years. The frequencies and the percentage of students, stratified by sex and age, are presented in Table 1. The prevalence of BP at baseline was 56% (51.7–60.2%); at the 3-year follow-up, the prevalence of BP significantly increased to 65.9% (61.8–69.8%; p < 0.001). This increase in the prevalence of BP at the follow-up was evident in both sexes. BP was significantly higher in girls than in boys at baseline (p = 0.011) and follow-up (p < 0.001; Figure 1). The incidence of BP in a 3-year period was 50.6% – from 231 students without BP at baseline and 117 developed BP after 3 years.
Table 1.
Frequencies and the percentage of students (n = 525) at baseline (2011) and at the 3-year follow-up, stratified by sex and age – Teutônia, Rio Grande do Sul, Brazil.
| Age (years) | Male n (%) |
Female n (%) |
Total N (%) |
|---|---|---|---|
| 11 | 24 (10.0) | 39 (13.7) | 63 (12.0) |
| 12 | 86 (35.8) | 91 (31.9) | 177 (33.7) |
| 13 | 60 (25.0) | 76 (26.7) | 136 (25.9) |
| 14 | 48 (20.0) | 60 (21.1) | 108 (20.6) |
| 15 | 18 (7.5) | 16 (5.6) | 34 (6.5) |
| 16 | 4 (1.7) | 3 (1.1) | 7 (1.3) |
| Total | 240 (100.0) | 285 (100.0) | 525 (100.0) |
Figure 1.
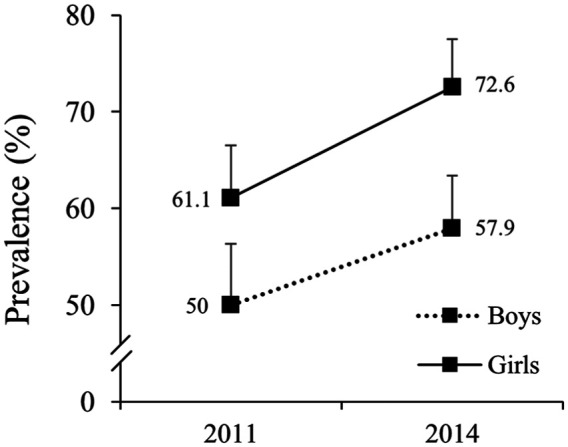
Prevalence of back pain in boys (n = 240) and girls (n = 285) at baseline (2011) and at the 3-year follow-up (2014) – Teutônia, Rio Grande do Sul, Brazil.
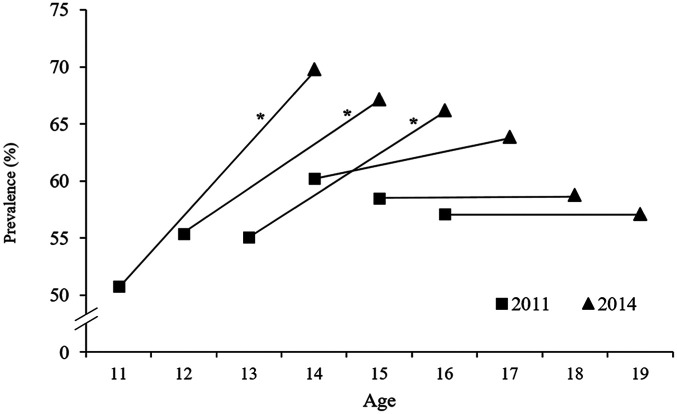
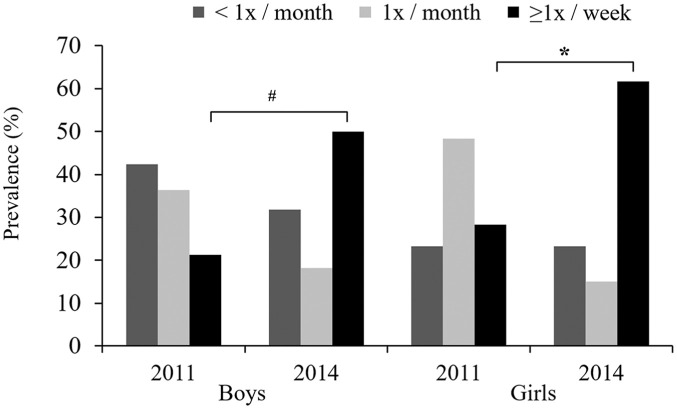
We detected a significant increase in BP prevalence in the 11- to 13-year-old students at the follow-up assessment (Figure 2). In 11-, 12- and 13-year-old students, the prevalence of BP compared to the baseline increased by 37.5%, 21.4% and 20% at the 3-year follow-up, respectively; in adolescents aged >13 years, the prevalence of BP showed a tendency of remaining unchanged between baseline and follow-up. A significant increase in the frequency with which BP was experienced (⩾1 time per week) was evident in both sexes at the 3-year follow-up evaluation (Figure 3).
Figure 2.
Prevalence of back pain in students (n = 525) at baseline (2011) and at the 3-year follow-up, stratified by age – Teutônia, Rio Grande do Sul, Brazil. *Significant increase from 2011 to 2014 (McNemar test, p < 0.05).
Figure 3.
Frequency of back pain in students at baseline (2011) and at the 3-year follow-up, stratified by sex – Teutônia, Rio Grande do Sul, Brazil. #Frequency increase in the response group ‘⩾1 time per week’ from 2011 to 2014 for boys (McNemar test, p = 0.002) and *frequency increase in the response group ‘⩾1 time per week’ from 2011 to 2014 for girls (McNemar test, p = 0.001).
Regarding the impact on day life activities, we found a significant increase (p < 0.001) in the prevention of daily life activities (i.e. playing, studying and practicing sports), from 13.9% (baseline) to 15% (follow-up). BP intensity increased after 3 years; however, no statistical differences were found between baseline and follow-up for both boys (baseline: 3.28 ± 1.90; 2014: 3.31 ± 1.97; p = 0.921) and girls (baseline: 3.42 ± 2.39; 2014: 3.78 ± 2.15; p = 0.165). Moreover, when we compared BP between sexes, girls presented higher (p = 0.033) intensity in the follow-up evaluation.
Most students were physically active and spent ⩾2 hours/day watching TV; at the same time, they used the computer, had parents with BP, were aware of their daily activities, had inadequate postures and carried a backpack on both shoulders (Table 2).
Table 2.
Back pain (outcome) and exposure variables (exercise, behavioural, hereditary and postural factors) – Teutônia, Rio Grande do Sul, Brazil (n = 525).
| Variables | Baseline (2011) |
Follow-up (2014) |
||
|---|---|---|---|---|
| N (%) | Back pain n (%) |
N (%) | Back pain n (%) |
|
| Exercise | ||||
| Physical exercise | ||||
| Yes | 476 (90.7) | 269 (56.5) | 463 (88.2) | 303 (65.4) |
| No | 49 (9.3) | 25 (51) | 62 (11.8) | 43 (69.4) |
| Physical exercise weekly frequencya (days/week) | ||||
| 1–2 | 233 (53.3) | 133 (59.6) | 190 (44.1) | 134 (70.5) |
| 3–4 | 143 (34.2) | 74 (51.7) | 172 (39.9) | 108 (62.8) |
| ⩾5 | 52 (12.4) | 29 (55.8) | 69 (16) | 40 (58) |
| Competitive exercisea | ||||
| Yes | 201 (42.7) | 106 (52.7) | 101 (22.1) | 67 (66.3) |
| No | 270 (57.3) | 158 (58.5) | 356 (77.9) | 232 (65.2) |
| Behavioural | ||||
| Time spent watching television per day (hours) | ||||
| 0–1 | 68 (15.2) | 38 (55.9) | 187 (39) | 127 (67.9) |
| 2–5 | 296 (66.1) | 151 (51) | 271 (56.6) | 168 (62) |
| ⩾6 | 84 (18.8) | 64 (76.2) | 21 (4.4) | 17 (81) |
| Time spent using computer per day (hours) | ||||
| 0–1 | 118 (29.6) | 60 (50.8) | 178 (39.2) | 125 (70.2) |
| 2–5 | 238 (59.8) | 135 (56.7) | 240 (52.9) | 150 (62.5) |
| ⩾6 | 42 (10.6) | 30 (71.4) | 36 (7.9) | 26 (72.2) |
| Time sleeping per night (hours/day) | ||||
| 0–7 | 125 (27.9) | 70 (56) | 282 (59.7) | 197 (69.9) |
| 8–9 | 258 (57.6) | 145 (56.2) | 175 (37.1) | 98 (56) |
| ⩾10 | 65 (14.5) | 36 (55.4) | 15 (3.2) | 12 (80) |
| Read and/or study in bed | ||||
| No | 82 (15.6) | 38 (46.3) | 124 (23.8) | 76 (61.3) |
| Sometimes | 276 (52.6) | 149 (54) | 210 (40.2) | 138 (65.7) |
| Yes | 167 (31.8) | 107 (64.1) | 188 (36) | 129 (68.6) |
| Hereditary | ||||
| Parents with back pain | ||||
| No | 158 (37.8) | 60 (38) | 133 (31.1) | 61 (45.9) |
| Yes | 260 (62.2) | 170 (65.4) | 295 (68.9) | 218 (73.9) |
| Postural | ||||
| Sleeping | ||||
| Supine | 35 (7.3) | 17 (48.6) | 47 (9.7) | 29 (61.7) |
| Lateral decubitus | 319 (66.3) | 169 (53) | 262 (53.8) | 168 (64.1) |
| Prone | 127 (26.4) | 81 (63.8) | 178 (36.6) | 121 (68) |
| Sitting posture to write | ||||
| Adequate | 79 (15) | 39 (49.4) | 33 (6.3) | 19 (57.6) |
| Inadequate | 446 (85) | 255 (57.2) | 487 (93.7) | 323 (66.3) |
| Sitting posture on a bench | ||||
| Adequate | 71 (13.5) | 38 (53.5) | 28 (5.4) | 17 (60.7) |
| Inadequate | 454 (86.5) | 256 (56.4) | 495 (94.6) | 328 (66.3) |
| Sitting posture to use computer | ||||
| Adequate | 119 (22.8) | 56 (47.1) | 70 (13.4) | 41 (58.6) |
| Inadequate | 404 (77.2) | 236 (58.4) | 452 (86.6) | 303 (67) |
| Posture to lift object from floor | ||||
| Adequate | 41 (7.9) | 25 (61) | 85 (16.3) | 53 (62.4) |
| Inadequate | 480 (92.1) | 266 (55.4) | 438 (83.7) | 292 (66.7) |
| Way to carry backpack | ||||
| Adequate (on both shoulders) | 441 (89.5) | 244 (55.3) | 351 (68.3) | 217 (61.8) |
| Inadequate (asymmetric) | 52 (10.5) | 36 (69.2) | 163 (31.7) | 120 (73.6) |
Related only to students in whom the variable was applicable.
The bivariate analysis indicated behavioural, hereditary and postural variables as risk factors for BP (Table 3). However, after these variables were included in the multivariate analysis and adjusted by age and sex, the variables that remained significantly associated with BP were the following: spending ⩾6 hours/day watching television, having parents with BP and asymmetrically carrying a backpack (inadequate posture) (Table 3). For those who spent much time watching TV (⩾6 hours/day) and did not have BP at baseline, the incidence of BP was 55%, while those who spend ⩽5 hours watching TV had a BP incidence of 48.6%. For those students without BP at baseline who had parents with BP, the incidence of BP was 54.4%, while for those with parents who did not have BP, the incidence was 40.8%. Moreover, for students who carried backpacks by an inadequate mode and did not have BP at the baseline, the incidence of BP was 62.5%, while those who carried the backpack by an adequate mode had a smaller BP incidence (49.7%).
Table 3.
Association between back pain (outcome) and exposure variables (exercise, behaviour, heredity and posture), depicted as relative risk – Teutônia, Rio Grande do Sul, Brazil (n = 525).
| Variables | Bivariate model Unadjusted RR (95% CI) |
p a | Multivariate model Adjusted RR (95% CI) |
p a |
|---|---|---|---|---|
| Control variables | ||||
| Age (baseline; years) | ||||
| 11–12 | 1 | 0.929 | ||
| 13–14 | 1.00 (0.96–1.05) | |||
| 15–16 | 0.98 (0.90–1.07) | |||
| Sex | ||||
| Male | 1 | <0.001 | 1 | <0.001 |
| Female | 1.08 (1.04–1.13) | 1.08 (1.04–1.13) | ||
| Exposure variables | ||||
| Exercise | ||||
| Physical exercise | ||||
| Yes | 1 | 0.946 | – | |
| No | 1.00 (0.94–1.07) | – | ||
| Physical exercise weekly frequencyb (days/week) | ||||
| 1–2 | 1 | 0.116 | – | |
| 3–4 | 0.96 (0.91–1.01) | – | ||
| ⩾5 | 0.95 (0.90–1.01) | – | ||
| Competitive exerciseb | ||||
| Yes | 1 | 0.156 | – | |
| No | 1.03 (0.99–1.08) | – | ||
| Behaviour | ||||
| Time spent watching television per day (hours) | ||||
| 0–1 | 1 | <0.001 | 1 | 0.002 |
| 2–5 | 0.95 (0.91–0.99) | 0.96 (0.92–1.01) | ||
| ⩾6 | 1.08 (1.01–1.14) | 1.07 (1.01–1.13) | ||
| Time spent using computer per day (hours) | ||||
| 0–1 | 1 | 0.094 | 1 | 0.115 |
| 2–5 | 0.98 (0.94–1.03) | 1.00 (0.96–1.05) | ||
| ⩾6 | 1.06 (0.97–1.13) | 1.06 (0.99–1.14) | ||
| Time sleeping per night (hours/day) | ||||
| 0–7 | 1 | 0.018 | 1 | 0.152 |
| 8–9 | 0.94 (0.90–0.98) | 0.96 (0.92–1.01) | ||
| ⩾10 | 0.97 (0.90–1.04) | 0.96 (0.89–1.04) | ||
| Read and/or study in bed | ||||
| No | 1 | 0.019 | 1 | 0.500 |
| Sometimes | 1.02 (0.97–1.08) | 1.01 (0.96–1.07) | ||
| Yes | 1.07 (1.02–1.13) | 1.03 (0.98–1.09) | ||
| Hereditary | ||||
| Parents with back pain | ||||
| No | 1 | <0.001 | 1 | <0.001 |
| Yes | 1.20 (1.14–1.26) | 1.16 (1.10–1.23) | ||
| Postural | ||||
| Sleeping | ||||
| Supine | 1 | 0.059 | 1 | 0.215 |
| Lateral decubitus | 1.01 (0.94–1.09) | 1.04 (0.96–1.12) | ||
| Prone | 1.06 (0.98–1.15) | 1.06 (0.98–1.14) | ||
| Sitting posture to write | ||||
| Adequate | 1 | 0.049 | 1 | 0.539 |
| Inadequate | 1.07 (1.01–1.14) | 1.02 (0.96–1.09) | ||
| Sitting posture on a bench | ||||
| Adequate | 1 | 0.274 | – | |
| Inadequate | 1.04 (0.97–1.11) | – | ||
| Sitting posture to use computer | ||||
| Adequate | 1 | 0.005 | 1 | 0.503 |
| Inadequate | 1.08 (1.02–1.13) | 1.02 (0.97–1.07) | ||
| Posture to lift object from floor | ||||
| Adequate | 1 | 0.808 | – | |
| Inadequate | 0.99 (0.94–1.05) | – | ||
| Way to carry backpack | ||||
| Adequate (symmetrical on both shoulders) | 1 | <0.001 | 1 | 0.037 |
| Inadequate (asymmetric) | 1.09 (1.05–1.13) | 1.04 (1.01–1.08) | ||
CI: confidence interval.
Generalized estimating equations were used to perform a Poisson regression model with robust variance errors. We used relative risk (RR) with their respective 95% CIs to measure effects (α = 0.05). The multivariate analysis was adjusted for sex and age and included the exposure variables (p < 0.10 in the bivariate analysis). Bold data reflect statistical significance (p < 0.05).
Significant association (p < 0.05).
Related only to students in whom the variable was applicable.
Discussion
To the best of our knowledge, this study is the first to evaluate BP prevalence and its risk factors in Latin America adolescents using a prospective study protocol. Our results showed a prevalence of BP ranging from 56% (baseline) to 66% (3-year follow-up), which is higher than that reported in other studies.35 The prevalence of BP increased by approximately 10% within a 3-year period. Similar findings were reported by Swain et al.27 who demonstrated that pain increased with age in adolescents aged 11–15 years from 28 countries in Europe, North America and Israel. We observed that the prevalence of BP significantly increased in the 11- to 13-year-old adolescents after 3 years, while we saw a tendency of an unchanged prevalence in adolescents aged >14 years.
The increase in the prevalence of BP during the study period was associated with an increase in age, which coincides with the pubertal growth spurt. Similarly, a longitudinal study in adolescents (11–13 years old) from Denmark26 reported an increase in the prevalence from 35.9% (baseline) to 48.5% (2 years later). Considering that puberty and BP are associated, and that this association is not only the result of an accumulation of other risk factors correlated with increasing age, this suggests a causal connection between puberty and BP.7 Importantly, we found that the prevalence of BP tended to stabilize after the age of 13 years and remained constant until the age of 19 years, implying that the prevalence of BP during adolescence may be an important predictor of BP into adulthood.27 Accordingly, the most effective time for interventions aimed at minimizing these findings is during puberty.36–38
Although we verified a similar increase in BP prevalence at follow-up in both boys and girls, as has been shown in previous studies,10,27,39 we also found that females had a higher risk of developing BP. It has been speculated that hormonal and biochemical mechanisms may contribute to higher rates of BP in girls.40
We also detected significant differences in the frequency with which BP was experienced over time. At the follow-up evaluation, the number of individuals who experienced BP ⩾1 time per week was more than double when compared to the baseline evaluation. Moreover, the BP impact on student life was higher at the follow-up. This shows that not only were more students affected by BP at the follow-up, as represented by the increased prevalence of BP, but they also experienced BP with greater frequency, and it had a greater impact over time. Furthermore, O’Sullivan et al.3 showed that BP may lead to negative impacts such as medication use and school absenteeism. Similar to our findings, BP consequences are generally greater among girls than among boys.3
Regarding the risk factors for BP, our multivariate analysis showed that watching television for ⩾6 hours/day, having parents with BP and carrying a school backpack asymmetrically were associated with a higher risk of developing BP. Sedentary activities have previously been documented as risk factors for the development of BP.18,41,42 Extended periods of sitting combined with physical inactivity can lead to a decrease in the nutrition of the joints and intervertebral discs, accelerating the degeneration of musculoskeletal structures.41 Moreover, watching television can involve inappropriate postural habits, which can also contribute to the development of BP.43,44 Meziat Filho et al.44 identified that youths who watched television with a slumped posture had a 3.22-fold increased risk of presenting with chronic BP.
Although hereditary factors for the development of BP have been investigated in recent years, no consensus exists in literature.40 Bejia et al.17 found an association between BP in 622 youths aged 11–19 years and a history of pain in their parents. Similarly, Kaspiris et al.45 found that the BP was 1.6 times greater among children with a family history of BP when compared to those without a family history. We also found that BP in parents or guardians was related to an increased BP risk in schoolchildren. Future studies should investigate the mechanisms by which this association can be explained. It has been shown that during childhood and adolescence, environmental factors exert more influence, while genetic factors play a limited role.45,46
School backpacks are the most frequent form of transportation of school materials.47 However, the way backpacks are loaded can directly affect the health of the schoolchildren.48,49 It has been shown that using only one strap may lead to postural changes50 that can alter the spine’s ability to absorb loads and can generate muscle spasms.51 Therefore, backpacks should be carried symmetrically (i.e. using both straps). In our study, carrying a backpack asymmetrically increased the risk of developing BP (RR = 1.04; 95% CI = 1.01–1.08). This finding demonstrates that, in addition to the widespread problem of school backpack weight,47,52 the way the backpack is loaded also merits attention.
BP in children and adolescents has been widely discussed in recent years,10,40 and some studies showed that BP during youth was predictive of BP in adulthood.27,53 However, to the best of our knowledge, all previous studies conducted in Latin America had a cross-sectional study design, making it impossible to establish cause-and-effect relationships.42,54 In this respect, longitudinal studies are important to identify the risk factors for the development of BP over time. We believe that information derived from a longitudinal study can inform future guidelines and aid in the search for early preventive measures for this public health problem.3,53
Our findings indicate that it is necessary to invest in preventive measures for BP and suggest that such interventions should occur during puberty, when there is the greatest progression of BP. One main limitation of this study relates to self-reporting as students were likely able to remember the past evaluation; however, the length of the follow-up interval (3 years) minimized the risk of students remembering. Moreover, the ‘frequency of physical exercise’ item of the questionnaire was not confirmed with an objective method such as an accelerometer. Another limitation is the lack of investigation regarding BP impact on school absence. For future research, we suggest that it is essential to include school absence as an important variable for the analysis.
In summary, we show that the prevalence of BP increased significantly over 3 years in adolescents aged ⩽13 years, stabilized in those aged >13 years and was higher among girls. The proportion of students who experienced BP at a higher frequency also increased over the study period, with more than double the number of schoolchildren experiencing BP with a frequency of ⩾1 time per week at the 3-year follow-up when compared to baseline. Having parents with BP, watching television for lengthy periods every day and carrying a backpack asymmetrically were risk factors for BP.
Footnotes
Authors’ contribution: MN, CTC and BNdR acquired the data. MN, CTC, AV and JFL analysed the data and performed the statistical analysis. MN and BNdR prepared the tables and the figures. All authors conceived and designed the study. They wrote the manuscript, revised it critically and read and approved the final manuscript.
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: This study was approved by the Ethics Research Committee from the Federal University of Rio Grande do Sul (number 19832).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: National Council of Technological and Scientific Development (CNPq – http://cnpq.br/), the Coordination of Improvement of Higher Education Personnel (CAPES – http://www.capes.gov.br/) and the Instituto Federal Goiano.
Guarantor: MN and CTC are guarantors of this article.
Informed consent: Adolescent consented to participate in our study as well as their guardians signed the written consent form to participate in the study.
ORCID iDs: Matias Noll  https://orcid.org/0000-0002-1482-0718
https://orcid.org/0000-0002-1482-0718
Adriane Vieira  https://orcid.org/0000-0003-3846-0873
https://orcid.org/0000-0003-3846-0873
References
- 1. Kongsted A, Kent P, Axen I, et al. What have we learned from ten years of trajectory research in low back pain? BMC Musculoskelet Disord 2016; 17(1): 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez M, Boyle E, Hartvigsen J, et al. Is this back pain killing me? All-cause and cardiovascular-specific mortality in older Danish twins with spinal pain. Eur J Pain 2017; 21(5): 938–948. [DOI] [PubMed] [Google Scholar]
- 3. O’Sullivan PB, Beales DJ, Smith AJ, et al. Low back pain in 17 year olds has substantial impact and represents an important public health disorder: a cross-sectional study. BMC Public Health 2012; 12(1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maher CG, Underwood M, Buchbinder R. Non-specific low back pain. Lancet 2017; 389: 736–747. [DOI] [PubMed] [Google Scholar]
- 5. Twiddy H, Hanna J, Haynes L. Growing pains: understanding the needs of emerging adults with chronic pain. Br J Pain 2017; 11(3): 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calvo-Muñoz I, Gómez-Conesa A, Sánchez-Meca J. Prevalence of low back pain in children and adolescents: a meta-analysis. BMC Pediatr 2013; 13(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lardon A, Leboeuf-Yde C, Le Scanff C, et al. Is puberty a risk factor for back pain in the young? A systematic critical literature review. Chiropr Man Therap 2014; 22(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah SA, Saller J. Evaluation and diagnosis of back pain in children and adolescents. J Am Acad Orthop Surg 2016; 24(1): 37–45. [DOI] [PubMed] [Google Scholar]
- 9. MacDonald J, Stuart E, Rodenberg R. Musculoskeletal low back pain in school-aged children. JAMA Pediatr 2017; 171(3): 280–287. [DOI] [PubMed] [Google Scholar]
- 10. Kamper SJ, Henschke N, Hestbaek L, et al. Musculoskeletal pain in children and adolescents. Braz J Phys Ther 2016; 20(3): 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leboeuf-Yde C, Kyvik KO. At what age does low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine 1998; 23(2): 228–234, http://www.ncbi.nlm.nih.gov/pubmed/9474731 [DOI] [PubMed] [Google Scholar]
- 12. Whittfield J, Legg SJ, Hedderley DI. Schoolbag weight and musculoskeletal symptoms in New Zealand secondary schools. Appl Ergon 2005; 36(2): 193–198. [DOI] [PubMed] [Google Scholar]
- 13. Trigueiro MJ, Massada L, Garganta R. Back pain in Portuguese schoolchildren: prevalence and risk factors. Eur J Public Health 2013; 23(3): 499–503. [DOI] [PubMed] [Google Scholar]
- 14. Grimmer K, Williams M. Gender-age environmental associates of adolescent low back pain. Appl Ergon 2000; 31(4): 343–360. [DOI] [PubMed] [Google Scholar]
- 15. Gunzburg R, Balague F, Nordin M, et al. Low back pain in a population of school children. Eur Spine J 1999; 8(6): 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato T, Ito T, Hirano T, et al. Low back pain in childhood and adolescence: a cross-sectional study in Niigata city. Eur Spine J 2008; 17(11): 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bejia I, Abid N, Ben Salem K, et al. Low back pain in a cohort of 622 Tunisian schoolchildren and adolescents: an epidemiological study. Eur Spine J 2005; 14(4): 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dolphens M, Vansteelandt S, Cagnie B, et al. Multivariable modeling of factors associated with spinal pain in young adolescence. Eur Spine J 2016; 25(9): 2809–2821. [DOI] [PubMed] [Google Scholar]
- 19. Noll M, Silveira EA, Avelar IS. Evaluation of factors associated with severe and frequent back pain in high school athletes. PLoS ONE 2017; 12(2): e0171978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarabottolo CC, Pinto RZ, Oliveira CB, et al. Back and neck pain prevalence and their association with physical inactivity domains in adolescents. Eur Spine J 2017; 26(9): 2274–2280. [DOI] [PubMed] [Google Scholar]
- 21. Auvinen J, Eskola PJ, Ohtonen H, et al. Long-term adolescent multi-site musculoskeletal pain is associated with psychological distress and anxiety. J Psychosom Res 2017; 93: 28–32. [DOI] [PubMed] [Google Scholar]
- 22. Szpalski M, Gunzburg R, Balagué F, et al. A 2-year prospective longitudinal study on low back pain in primary school children. Eur Spine J 2002; 11(5): 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franz C, Wedderkopp N, Jespersen E, et al. Back pain in children surveyed with weekly text messages–a 2.5 year prospective school cohort study. Chiropr Man Therap 2014; 22(1): 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mueller S, Mueller J, Stoll J, et al. Incidence of back pain in adolescent athletes: a prospective study. BMC Sports Sci Med Rehabil 2016; 8(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamada M, Abe T, Kitayuguchi J, et al. Dose–response relationship between sports activity and musculoskeletal pain in adolescents. Pain 2016; 157(6): 1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aartun E, Hartvigsen J, Wedderkopp N, et al. Spinal pain in adolescents: prevalence, incidence, and course: a school-based two-year prospective cohort study in 1, 300 Danes aged 11–13. BMC Musculoskelet Disord 2014; 15(1): 187–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swain M, Henschke N, Kamper S, et al. An international survey of pain in adolescents. BMC Public Health 2014; 14(1): 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa Nacional por Amostra de Domicílios (Síntese dos Indicadores Sociais 2009). Rio de Janeiro: IBGE, 2010. [Google Scholar]
- 29. Noll M, Candotti CT, da Rosa BN, et al. High prevalence of inadequate sitting and sleeping postures: a three-year prospective study of adolescents. Sci Rep 2017; 7(1): 14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noll M, Tarragô Candotti C, Vieira A, et al. Back pain and body posture evaluation instrument (BackPEI): development, content validation and reproducibility. Int J Public Health 2013; 58(4): 565–572. [DOI] [PubMed] [Google Scholar]
- 31. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159(7): 702–706. [DOI] [PubMed] [Google Scholar]
- 32. Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods 2004; 7(2): 127–150. [Google Scholar]
- 33. Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013; 22(6): 661–670. [DOI] [PubMed] [Google Scholar]
- 34. Brady SRE, Hussain SM, Brown WJ, et al. Relationships between weight, physical activity, and back pain in young adult women. Medicine 2016; 95(19): e3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain 2011; 152(12): 2729–2738. [DOI] [PubMed] [Google Scholar]
- 36. Dolphens M, Cagnie B, Danneels L, et al. Long-term effectiveness of a back education programme in elementary schoolchildren: an 8-year follow-up study. Eur Spine J 2011; 20(12): 2134–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Candotti CT, Rohr JE, Noll M. Postural education as curriculum content of physical education in elementary education schools in the city of Montenegro/RS. Movimento 2011; 17(3): 57–77. [Google Scholar]
- 38. Noll M, Vieira A, Darski C, et al. Back schools in Brazil: a review of the intervention methodology, assessment tools, and results. Rev Bras Reumatol 2014; 54(1): 51–58. [PubMed] [Google Scholar]
- 39. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012; 64(6): 2028–2037. [DOI] [PubMed] [Google Scholar]
- 40. Stanford EA, Chambers CT, Biesanz JC, et al. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain 2008; 138(1): 11–21. [DOI] [PubMed] [Google Scholar]
- 41. Paananen MV, Auvinen JP, Taimela SP, et al. Psychosocial, mechanical, and metabolic factors in adolescents’ musculoskeletal pain in multiple locations: a cross-sectional study. Eur J Pain 2010; 14(4): 395–401. [DOI] [PubMed] [Google Scholar]
- 42. Fernandes JAA, Genebra CVDS, Maciel NM, et al. Low back pain in schoolchildren: a cross-sectional study in a western city of São Paulo state, Brazil. Acta Ortop Bras 2015; 23(5): 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minghelli B, Oliveira R, Nunes C. Non-specific low back pain in adolescents from the south of Portugal: prevalence and associated factors. J Orthop Sci 2014; 19(6): 883–892. [DOI] [PubMed] [Google Scholar]
- 44. Meziat Filho N, Coutinho ES, Azevedo e Silva G. Association between home posture habits and low back pain in high school adolescents. Eur Spine J 2015; 24(3): 425–433. [DOI] [PubMed] [Google Scholar]
- 45. Kaspiris A, Grivas TB, Zafiropoulou C, et al. Nonspecific low back pain during childhood: a retrospective epidemiological study of risk factors. J Clin Rheumatol 2010; 16(2): 55–60. [DOI] [PubMed] [Google Scholar]
- 46. El-Metwally A, Mikkelsson M, Ståhl M, et al. Genetic and environmental influences on non-specific low back pain in children: a twin study. Eur Spine J 2008; 17(4): 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mwaka ES, Munabi IG, Buwembo W, et al. Musculoskeletal pain and school bag use: a cross-sectional study among Ugandan pupils. BMC Res Notes 2014; 7(1): 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adeyemi AJ, Rohani JM, Abdul Rani MR. Backpack-back pain complexity and the need for multifactorial safe weight recommendation 2017; 58: 573–582. [DOI] [PubMed] [Google Scholar]
- 49. Neuschwander TB, Cutrone J, Macias BR, et al. The effect of backpacks on the lumbar spine in children: a standing magnetic resonance imaging study. Spine 2009; 35(1): 83–88. [DOI] [PubMed] [Google Scholar]
- 50. Noll M, de Avelar IS, Lehnen GC, et al. Back pain prevalence and its associated factors in Brazilian athletes from public high schools: a cross-sectional study. PLoS ONE 2016; 11(3): e0150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goodgold S, Corcoran M, Gamache D, et al. Backpack use in children. Pediatr Phys Ther 2002; 14(3): 122–131. [DOI] [PubMed] [Google Scholar]
- 52. Spiteri K, Busuttil ML, Aquilina S, et al. Schoolbags and back pain in children between 8 and 13 years: a national study. Br J Pain 2017; 11(2): 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burton AK, Balague F, Cardon G, et al. Chapter 2: European guidelines for prevention in low back pain. Eur Spine J 2006; 15(Suppl. 2): S136–S168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silva GRR, Pitangui ACR, Xavier MKA, et al. Prevalence of musculoskeletal pain in adolescents and association with computer and videogame use. J Pediatr 2016; 92(2): 188–196. [DOI] [PubMed] [Google Scholar]