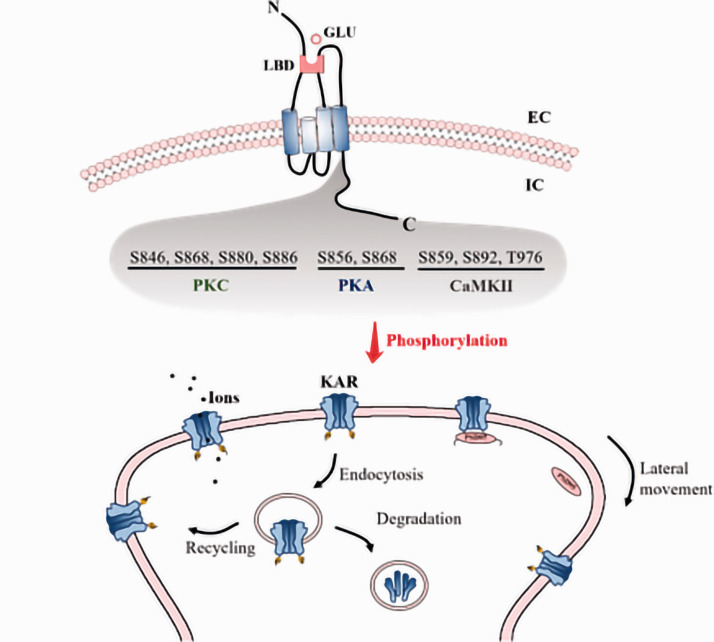
Figure 2.
Post-translational modifications (phosphorylation) of KA receptors. A number of residues within KA receptor subunits were shown to be phosphorylated, including S846/S868/S880/S886 (PKC), S856/S868 (PKA), S859/S892/T976 (CaMKII). All of these phosphorylation is shown to directly impact the kainite-evoked currents, and is also illustrated to be related to the endocytosis of KA receptors, leading to recycling of KA receptors to the membrane or degradation. Moreover, phosphorylation uncouples KA receptors from postsynaptic density 95 (PSD-95), improving the overall lateral mobility of these receptors by freeing them from synaptic incorporation. LBD ligand binding domain, GLU glutamate, N N-terminal domain, C C-terminal domain, P phosphorylation, EC extracellular, IC intracellular.