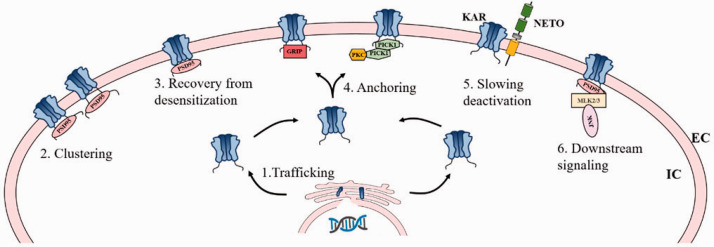
Figure 3.
Schematic of domain organization of PSD95, GRIP, PICK1, and Netos with KA receptors. PDZ domain proteins (PSD95, GRIP and PICK1) are shown to interact with KA receptors through the PDZ domain-mediated interactions, which are necessary for the appropriate regulation of KA receptor-mediated synaptic functionality. Interactions with PSD95 enable KA receptors to recover more rapidly following receptor desensitization. Such interactions also drive KA receptor clustering, and KA receptors-PSD95 complex can additionally interact with mixed-lineage kinases 2 and 3 (MLK2 and MLK3), which drives JNK kinase activation. GRIP regulates KA receptor anchoring at synapses, and that the PICK1-targeted phosphorylation of KA receptors by PKC stabilizes GRIP binding. NETOs (NETO1/2) interact with KA receptors via their PDZ-ligand domains and CUB domains, thereby forming stable complexes with KA receptors. Netos interaction with KA receptors typically slow KA receptor deactivation kinetics, and such interactions also represents a regulator of KA receptor trafficking. EC extracellular, IC intracellular.