Figure 6. ESM1 associates with the ARM domain of β‐catenin.
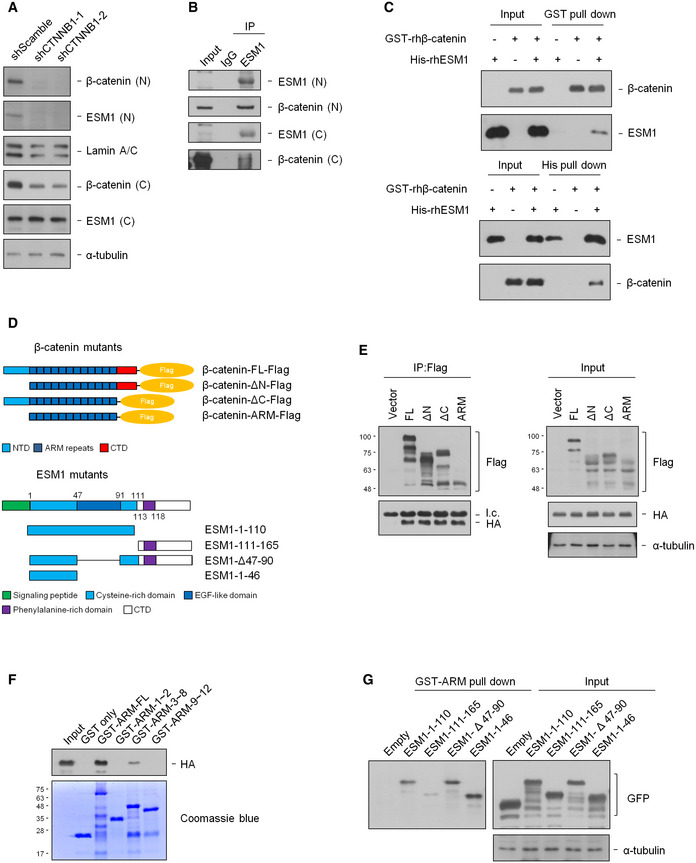
- Nuclear and cytosolic extracts of 22Rv1‐M cells transfected with either shScramble or shCTNNB1 were prepared, and the levels of indicated proteins were detected by immunoblotting.
- Nuclear or cytosolic extracts of 22Rv1‐M cells were immunoprecipitated with an ESM1 antibody.
- Human recombinant His‐ESM1 and GST‐β‐catenin proteins were pull down with either Ni sepharose or glutathione sepharose.
- Upper, mapping β‐catenin regions binding to ESM1. Schematic diagram of full‐length β‐catenin and deletion mutants. Lower, mapping ESM1 regions binding to β‐catenin. Schematic diagram of full‐length ESM1 and deletion mutants.
- HEK293T cells were co‐transfected with the indicated β‐catenin‐Flag and ESM1‐HA constructs. Cell extracts were immunoprecipitated with Flag‐M2 agarose beads. Light chain was labeled as l.c.
- HEK293T cells were transfected with ESM1‐HA constructs, and pull‐down was carried out with different purified GST‐ARM fragments.
- HEK293T cells were transfected with the indicated ESM1‐GFP constructs. Cell extracts were pulled down with purified GST‐ARM.
Source data are available online for this figure.
