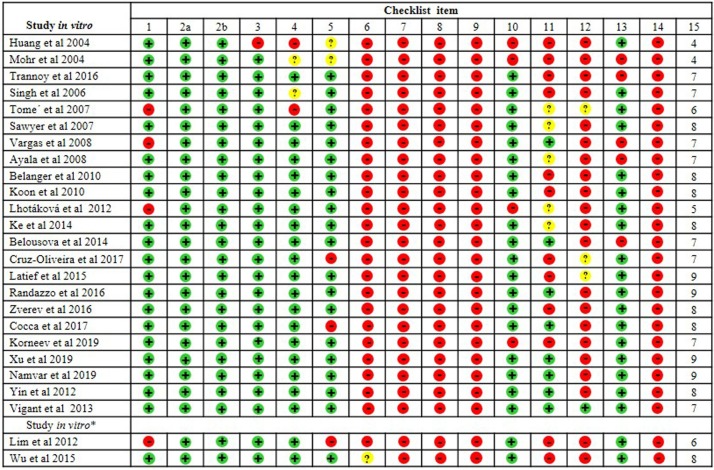
Fig. 2.
Risk of bias of in vitro studies' according to the modified CONSORT checklist. *In two trial both methods (in vitro and in vitro studies) were investigated. *Part A (in vitro studies). 1) Structured abstract; 2a) Scientific background and rationale; 2b) Objectives and/or hypotheses; 3) Intervention of each group; 4) Outcomes; 5) Sample size; 6) Randomization: sequence generation; 7) Allocation concealment mechanism; 8) Implementation; 9) Blinding; 10) Statistical methods; 11) Outcomes and estimation; 12) Limitations; 13) Funding; 14) Protocol; 15) Scores. (+) Low risk of bias; (-) High risk of bias; (?) Unclear risk of bias. *.