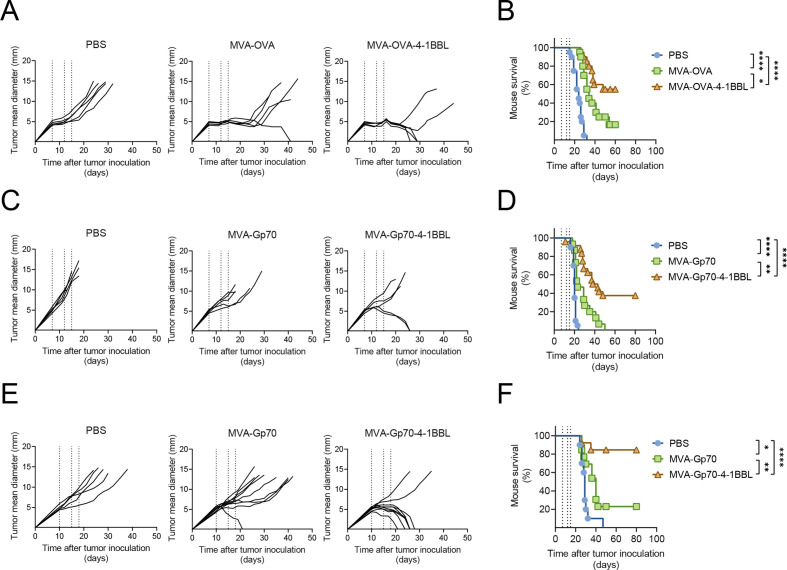
Figure 1.
Therapeutic efficacy of intratumoral (IT) administration of MVA-TAA-4-1BBL in unrelated tumor models is independent of the choice of antigen. C57BL/6 (A–D) or Balb/c mice (E, F) received either 5×105 B16.OVA (A, B), 5×105 B16.F10 (C, D) or 5×105 CT26.WT (E, F) cells subcutaneously (SC) in the flank. 7–14 days later, when tumor volumes were above 60 mm3, mice were immunized intratumorally (IT) either with phosphate buffered saline (PBS) or with the indicated MVA constructs. IT immunization was repeated on days 4 or 5 and 8 after the first immunization (dotted lines). (A) Tumor size follow-up (n=5 mice/group) and (B) overall survival (n=20 mice/group) of B16.OVA bearing mice injected either with PBS, 2×108 TCID50 MVA-OVA or 2×108 TCID50 MVA-OVA-4-1BBL; (C) tumor size follow-up (n=5 mice/group) and (D) overall survival (n=15 mice/group) of B16.F10 bearing mice injected either with PBS, 5×107 TCID50 MVA-Gp70 or 5×107 TCID50 MVA-Gp70-4-1BBL; (E) tumor size follow-up (n=5 mice/group) and (F) overall survival (n=10 mice/group) of CT26.WT bearing mice injected either with PBS, 5×107 TCID50 MVA-Gp70 or 5×107 TCID50 MVA-Gp70-4-1BBL. (A, C, E) Data are representative of at least two independent experiments. (B, D, F) Represent overall survival of at least two merged independent experiments. Log-rank test on mouse survival was performed for figures B, D, F. *P<0.05; **p<0.01; ****p<0.0001. IT, intratumoral; MVA, modified vaccinia Ankara; OVA, ovalbumin; PBS, phosphate buffered saline; SC, subcutaneous.