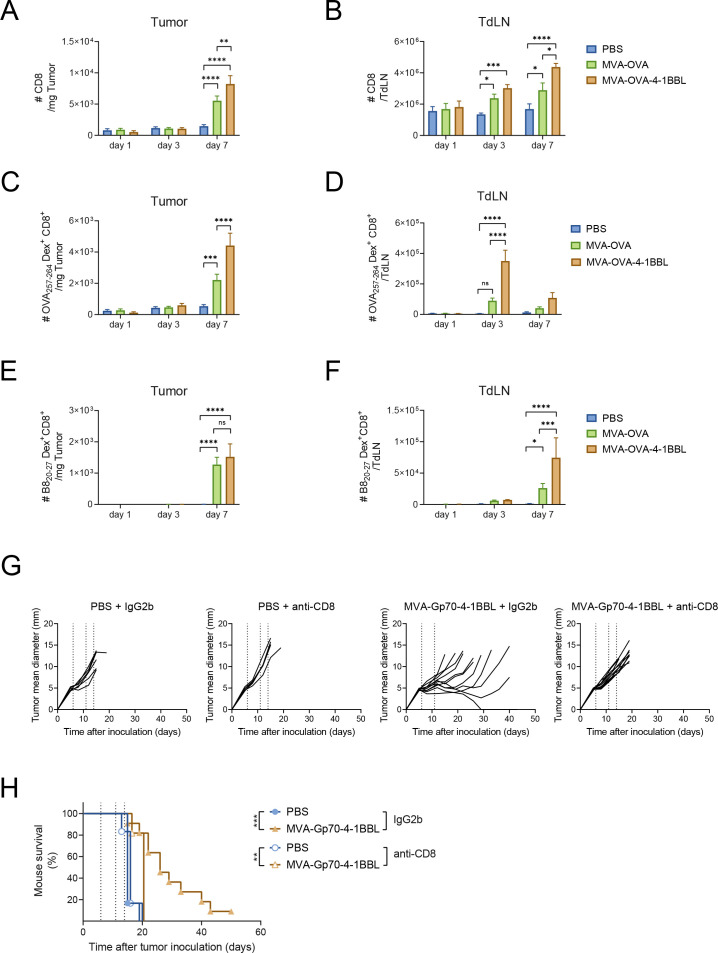
Figure 2.
CD8+ T cell expansion and dependency on IT MVA-OVA-4-1BBL. C57BL/6 mice received 5×105 B16.OVA cells subcutaneously in the flank. Ten days later when tumor volumes were around 80 mm3, mice were grouped and IT injected with either PBS, 2×108 TCID50 MVA-OVA or MVA-OVA-4-1BBL. 1, 3 and 7 days after immunization, mice were sacrificed for further analysis (n=5–11 mice/group). (A) Number of CD8+ T cells per Mg tumor; (B) number of CD8+ T cells per tumor-draining lymph node (TdLN); (C) number of OVA257-264 –specific CD8+ T cells per Mg tumor; (D) number of OVA257-264 –specific CD8+ T cells per TdLN; (E) Number of B820-27 –specific CD8+ T cells per Mg tumor; (F) number of B820-27 –specific CD8+ T cells per TdLN; (G, H) When B16.F10 tumor volumes were above 60 mm3, mice received PBS or were immunized IT with 5×107 TCID50 of MVA-Gp70-4-1BBL. IT immunization was repeated on day 5 and 8 after the first immunization (dotted lines). Mice received 200 µg of either IgG2b or anti-CD8 antibody intraperitoneally (IP) at day −2, 2, 6 and 10 after immunization; (G) tumor size follow-up (n=8 mice/group) and (H) overall survival (n=8 mice/group). Data in A–F expressed as mean±SEM A–F two-way ANOVA comparing cell numbers in analyzed organs on treatment. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Log-rank test on mouse survival was performed for (H). **P<0.01; ***p<0.001. ANOVA, analysis of variance; IP, intraperitoneally; IT, intratumoral; MVA, modified vaccinia Ankara; n.s., non-significant; OVA, ovalbumin; PBS, phosphate buffered saline; SC, subcutaneous; SEM, SE of the mean.