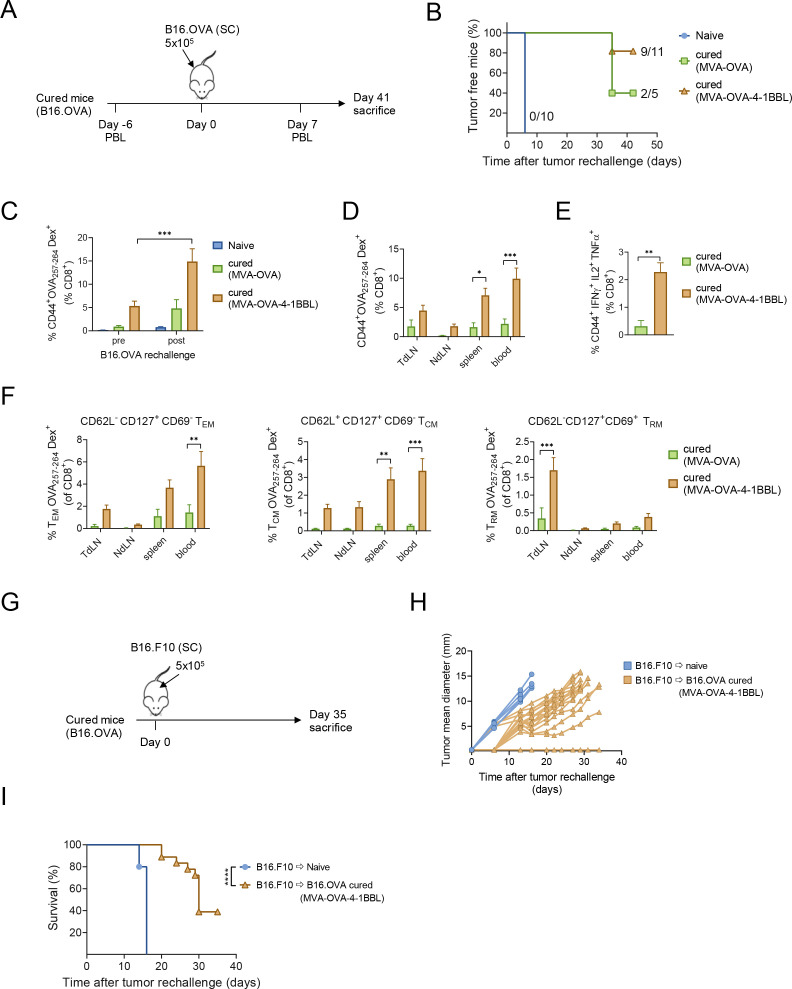
Figure 5.
IT MVA-TAA-4-1BBL treatment protects from local tumor rechallenge and induces epitope spreading. (A) Experimental layout. Naïve C57BL/6 mice or long-term survivors (12–36 weeks after tumor clearance) of figure 1A, B were rechallenged SC into the tumor-naïve flank of cured mice with 5×105 B16.OVA cells. Peripheral blood was analyzed by flow cytometry before (day −6) and after (day 7) after rechallenge. Blood, spleen, NdLN and TdLN mononuclear cells were analyzed on day 41 after tumor cell inoculation. (B) Percentage of tumor-free mice over time is displayed (n=5–11 mice/group). Number of tumor-free mice per group is shown. (C) Frequency of peripheral blood CD44+ OVA257-264 Dex+ CD8+ T cells pre-B16 and post-B16.OVA rechallenge of naïve mice and long-term survivors after IT MVA-OVA or MVA-OVA-4-1BBL treatment. (D) Frequency of CD44+ OVA257-264 Dex+ CD8+ T cells in blood, spleen, NdLN and TdLN. (E) Frequency of splenic CD44+ IFNγ+ TNFα+ IL2+ CD8+ T cells after restimulation with OVA257-264 peptide. (F) Frequency of CD62L- CD127+ CD69- OVA257-264 Dex+ T cells (TEM) in blood, spleen, NdLN and TdLN (left). Frequency of CD62L+ CD127+ OVA257-264 Dex+ cells (TCM) in blood, spleen, NdLN and TdLN (middle). Frequency of CD62L- CD127+ CD69+ OVA257-264 Dex+ (TRM) in blood, spleen, NdLN and TdLN 41 days after B16.OVA cell challenge (right). (G) Experimental layout. Naïve C57BL/6 mice (n=5) or long-term survivors (12–36 weeks after tumor clearance, n=18) that rejected B16. OVA tumors on IT MVA-OVA-4-1BBL were rechallenged SC into the left flank with 5×105 B16.F10 cells. (H) Tumor size follow-up. (I) Overall survival. data in A–H expressed as Mean±SEM. (C–F) Two-way ANOVA was performed. *P<0.05; **p<0.01; ***p<0.005. (E) one-way ANOVA was performed. *P<0.05; **p<0.01; ***p<0.001. Log-rank test on mouse survival was performed for figure 1. ***P<0.001. ANOVA, analysis of variance; IFNγ, interferon-γ; IL2, interleukin 2; IT, intratumoral; MVA, modified vaccinia Ankara; NdLN, non-draining lymph node; OVA, ovalbumin; PBL, peripheral blood lymphocyte; SC, subcutaneous; SEM, SE of the mean; TdLN, tumor-draining lymph node; TNFα, tumor necrosis factor-α; TRM, resident memory T cells.