Abstract
Background
Transdermal antipsychotic patch formulations offer potential benefits, including improved adherence. This study investigated the striatal dopamine D2 receptor occupancy with daily blonanserin transdermal patch application.
Methods
This open-label, phase II study enrolled 18 Japanese outpatients (20 to <65 years) with schizophrenia (DSM-IV-TR criteria; total Positive and Negative Syndrome Scale score <120 at screening) treated with blonanserin 8-mg or 16-mg tablets. Patients continued tablets for 2–4 weeks at their current dose and were then assigned to once-daily blonanserin patches (10/20/40/60/80 mg daily) for 2–4 weeks based on the oral dose. [11C]raclopride positron emission tomography scanning determined blonanserin striatal dopamine D2 receptor occupancy (primary endpoint). Secondary endpoints included assessment of receptor occupancy by dose, changes in Positive and Negative Syndrome Scale and Clinical Global Impressions-Severity of Illness-Severity scores, patient attitudes towards adherence, and patch adhesiveness.
Results
Of 18 patients who started the blonanserin tablet treatment period, 14 patients completed treatment. Mean D2 receptor occupancy for blonanserin tablets 8 mg/d (59.2%, n = 5) and 16 mg/d (66.3%, n = 9) was within the values for blonanserin patches: 10 mg/d (33.3%, n = 3), 20 mg/d (29.9%, n = 2), 40 mg/d (61.2%, n = 3), 60 mg/d (59.0%, n = 3), and 80 mg/d (69.9%, n = 3). Occupancy generally increased with increasing blonanserin dose for both formulations with the half maximal receptor occupancy for tablets and patches associated with doses of 6.9 mg/d and 31.9 mg/d, respectively. Diurnal variability in occupancy was lower during transdermal patch treatment than during tablet treatment. Blonanserin transdermal patches were well tolerated with no major safety concerns.
Conclusions
Blonanserin patches (40/80 mg/d) have lower diurnal variability in occupancy than blonanserin tablets (8/16 mg/d), and patches at doses of 40 mg/d and 80 mg/d appear to be a suitable alternative for blonanserin tablets at doses of 8 mg/d and 16 mg/d, respectively. Blonanserin patches represent a potential new treatment option for patients with schizophrenia.
Trial registry
JAPIC Clinical Trials Information registry (www.clinicaltrials.jp; JapicCTI-No: JapicCTI-121914).
Keywords: blonanserin, dopamine receptor occupancy, positron emission tomography, schizophrenia, transdermal patches
Significance Statement.
Transdermal patch formulations of antipsychotics offer the potential for improved tolerability and adherence for patients with schizophrenia provided that efficacy is similar to that of existing formulations. Blonanserin is an atypical antipsychotic with high affinity for dopamine D2, D3, and serotonin 5-HT2A receptors and a low potential for weight gain and hyperprolactinemia. This open-label, uncontrolled, phase II study using [11C]raclopride PET scanning to determine dopamine D2 receptor occupancy found that blonanserin patches at doses of 40 mg/d and 80 mg/d appear to be suitable alternatives for blonanserin tablets at doses of 8 mg/d and 16 mg/d, respectively. Subsequent phase III studies of blonanserin transdermal patches at these proposed doses have properly assessed the efficacy and safety of this formulation and its potential for patients with schizophrenia in clinical practice, especially for those with tolerability and adherence issues.
Introduction
Blonanserin (Lonasen, Sumitomo Dainippon Pharma Co., Ltd.) is an atypical antipsychotic approved for use in Japan, Korea, and China (Harvey et al., 2019; Iwata et al., 2020a). Blonanserin has a high affinity for dopamine D2, D3, and serotonin 5-HT2A receptors and is a full antagonist at dopamine D2/D3 receptors but has low affinity for dopamine D1, 5-HT1A, 5-HT3, α/β-adrenergic, histaminic, and muscarinic receptors (Deeks and Keating, 2010). In short- and long-term studies, blonanserin has been shown to be effective and well tolerated in patients with schizophrenia (Une and Kurumiya, 2007; Deeks and Keating, 2010; Baba et al., 2015; Li et al., 2015; Kishi et al., 2019). A meta-analysis of randomized controlled clinical trials found that blonanserin was more effective than aripiprazole in improving total Positive and Negative Syndrome Scale (PANSS) scores and was associated with a lower risk of hyperprolactinemia but had a higher risk of akathisia, agitation/excitement, and extrapyramidal symptoms compared with a combination of risperidone and paliperidone (Kishi et al., 2019). Open-label studies in Japan have found blonanserin to be effective and well tolerated over observation periods of up to 56 weeks (Murasaki, 2007; Kinoshita, 2008; Takahashi et al., 2013).
Transdermal patch formulations offer several potential advantages to patients with psychiatric illnesses (Citrome et al., 2019; Iwata et al., 2020b). In patients with schizophrenia, transdermal patch formulations of antipsychotics may improve tolerability by allowing more continuous plasma concentrations, fewer gastrointestinal adverse events, and lower effective doses via avoidance of first-pass metabolism. Transdermal patches may improve adherence by helping patients avoid multiple daily doses, adverse effects, undesirable administration routes (especially injections), and by allowing visual checks (Citrome et al., 2019; Iwata et al., 2020b), because poor adherence is a common and complex problem in patients with schizophrenia (Mohr and Volavka, 2012).
However, an important priority with transdermal patches and other alternative formulations is to reliably establish comparable efficacy with the existing formulation. Positron emission tomography (PET) studies are a useful objective tool in research of schizophrenia and treatment with antipsychotics. PET allows the investigation of in vivo cerebral dopamine neurotransmission, including dopaminergic parameters of D2 and D3 receptors, dopamine synthesis capacity and release, and transporters (Sekine et al., 2011; Howes et al., 2012). Of relevance to the present study, PET studies also allow determination of dopamine receptor occupancy and correlation with antipsychotic dose as well as clinical effects (Farde et al., 1988, 1992, 1995; Nordstrom et al., 1993; Ito et al., 1999). A previous PET study among Japanese patients with schizophrenia administered blonanserin tablets at clinical daily doses and used [11C]raclopride to demonstrate measured striatal dopamine D2 receptor occupancy at 4.5–6.5 hours after daily doses of 8 mg, 16 mg, and 24 mg of 60.8%, 73.4%, and 79.7%, respectively (Tateno et al., 2013). The extent of dopamine D2 receptor occupancy has been shown to contribute to the clinical effects of antipsychotics in terms of reductions in clinical scores such as PANSS (Yilmaz et al., 2012).
Based on the known therapeutic profile of blonanserin and the findings from the previous receptor occupancy study with the tablet formulation, the primary aim of the present study is to investigate the striatal dopamine D2 receptor occupancy associated with daily application of blonanserin transdermal patches, which are the first transdermal patches in the world approved for the treatment of schizophrenia. The study will also aim to investigate the relationship between dopamine D2 receptor occupancy and dose for blonanserin patches alone and compared with blonanserin tablets to determine the clinical dosage range (i.e., the range of doses to be verified in phase III studies). In addition, efficacy, safety, patient-reported outcomes and measures of patients’ attitude to treatment, and adhesion characteristics associated with the blonanserin patch formulation are investigated.
Methods
Study Design and Patients
This was an open-label, uncontrolled, phase II study primarily designed to determine the dopamine D2 receptor occupancy in the striatum after 2- to 4-week daily application of blonanserin transdermal patches. Secondary objectives of the study were designed to determine (1) the relationship between blonanserin dose and striatal dopamine D2 receptor occupancy after application of blonanserin patches and blonanserin tablets; (2) changes in total PANSS scores and Clinical Global Impressions-Severity of Illness scale (CGI-S) scores with blonanserin patches and tablets; and (3) the safety, patient preference, and adhesiveness characteristics of blonanserin patches. This study was registered in Japan with the JAPIC Clinical Trials Information registry (JapicCTI-No: JapicCTI-121 914).
The current study was conducted between August 2012 and May 2014. Eligible outpatients of the Nippon Medical School Hospital and affiliated clinics were included if they were aged 20 to <65 years with schizophrenia (according to DSM-IV-TR criteria), had a total PANSS score <120 at the screening visit, and were already treated with blonanserin tablets as monotherapy on an outpatient basis. Key exclusion criteria were contraindications to blonanserin treatment; current or history of neuroleptic malignant syndrome, tardive dyskinesia, water intoxication, Parkinson’s disease, suicide attempts or suicidal ideation, diabetes mellitus; severe cardiovascular, liver, blood, endocrine, or kidney disease as well as brain organic disease, serious brain abnormalities as detected by head MRI at screening, or convulsions; a history of drug or alcohol abuse/dependence (within 6 months before screening); and antipsychotic treatment via long-acting injection (within 3 months before screening), or electroconvulsive therapy (within 6 months before screening).
During the study treatment periods, most concomitant medications that were centrally acting or could block dopamine D2 receptors were prohibited or restricted, including antipsychotics (except for blonanserin), antimanic/antiepileptic drugs, digestive agents that affect dopamine receptors, adrenaline, and CYP3A4 inhibitors or inducers. In addition, antiparkinsonian drugs were ceased on request before screening, although concomitant anticholinergic agents were permitted, as judged necessary, for the treatment of new extrapyramidal symptoms that emerged during treatment.
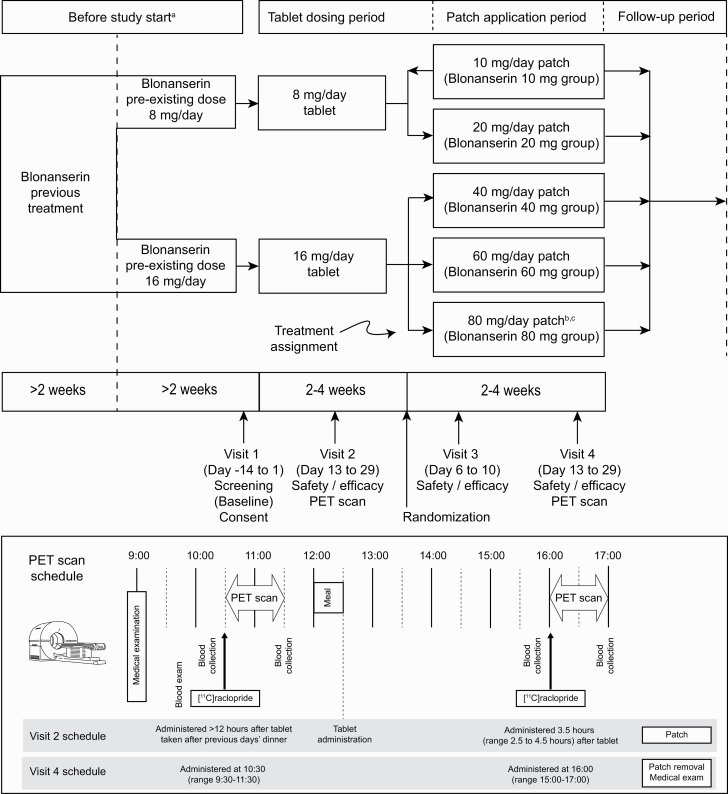
After obtaining informed consent, outpatients judged to be eligible at the screening visit (visit 1, baseline) entered the blonanserin tablet treatment period and received the same dose of blonanserin tablets (8 mg/d or 16 mg/d) as they had been taking before the study (Figure 1). After oral blonanserin administration for at least 2 (up to 4) weeks, PET scans and assessments of efficacy/safety were performed (visit 2).
Figure 1.
Study design. aSubjects receiving blonanserin for ≥4 weeks and blonanserin 4 mg or 8 mg twice daily (8 mg/d or 16 mg/d) for ≥2 weeks entered this study. bAn 80-mg/d blonanserin patch arm was added during a revision to the protocol. cNo randomization to 80-mg/d patch group (direct assignment).
Following the blonanserin tablet treatment period, patients were assigned to treatment with blonanserin patches at doses of 10 mg/d, 20 mg/d, 40 mg/d, and 60 mg/d in an open-label manner based on the dose (8 mg/d or 16 mg/d) used in the blonanserin tablet treatment period (Figure 1). Patients who received blonanserin tablets at 8 mg/d were randomly assigned to receive either 10 mg/d or 20 mg/d blonanserin patch treatment. Patients who received blonanserin tablets at 16 mg/d were either randomized to the 40-mg/d or 60-mg/d blonanserin patch groups. By protocol amendment, blonanserin patch 80-mg/d group were added, and allocation to 10 mg/d was stopped after enrolment of 3 patients, as the observed occupancy at this dose was low.
Blonanserin transdermal patches were attached to the back or chest at night after bathing, if taken. When blonanserin transdermal patches were replaced, they were applied to the same overall application site (back or chest) throughout the patch phase but in a different location from that of the previous application. After 1 week of blonanserin transdermal patch administration, clinical assessments were performed and, after blonanserin transdermal patch administration for at least 2 (up to 4) weeks, a second set of PET scans and further assessments of efficacy/safety were performed (visit 4).
This study was conducted in compliance with ethical principles based on the Declaration of Helsinki and in compliance with the “Ministerial Ordinance on Standards for Conducting Clinical Trials of Drugs” (Ministry of Health, Labour and Welfare No. 161, 28 December 2012). This study was approved by the institutional review board of the Nippon Medical School Hospital, Japan. Prior to the study, written informed consent was obtained from all participants after complete explanation of the study.
Endpoints and Assessments
Efficacy and Pharmacodynamic Endpoints
The primary endpoint was the proportion of striatal dopamine D2 receptors occupied by blonanserin (“receptor occupancy”) as determined by [11C]raclopride PET scanning.
For visit 2 and visit 4, patients were instructed to visit the clinic in a fasted state. On the day of the PET scan, [11C]raclopride was administered and the radioactivity distribution was measured by the first PET scan. Promptly following the first PET scan, a meal was followed by blonanserin tablet administration (visit 2 only) after which [11C]raclopride administration and a second PET scan were performed. If the synthesized [11C]raclopride did not meet the quality standards as a ligand, the PET scan could not be carried out as scheduled, but clinical assessments were still conducted on the day of the PET scan.
For visit 2, PET scans were timed to correlate with trough and peak times associated with twice daily oral administration of blonanserin. Accordingly, a trough PET scan was performed 12 hours or more since the last dose (10:30–11:30) while the peak PET scan was performed 3.5 hours after administration (16:00–17:00) in line with Tmax, which is approximately 3.8 hours after a mealtime dose. For visit 4, PET scans were timed to correlate with results of the repeated patch application tests, and target measurement times were set to 10:30 and 16:00, with a 1-hour deviation window, to match the time to expected Tmin and Tmax for the patches, respectively.
To determine the primary endpoint, the percentage of dopamine D2 receptor occupancy was calculated using the equation: (BPbasea—BPdrugb) / BPbase × 100 (%), where “a” is the dopamine D2 receptor binding potential (BP) in healthy adults and “b” is the dopamine D2 receptor BP during study drug administration. To obtain BPbase, dopamine D2 BP of [11C]raclopride was measured in 32 healthy volunteers aged 24 to 74 years. Distribution of dopamine D2 receptor BP was plotted by age and a regression line was obtained. Dopamine D2 receptor BPs corresponding to the age of each patient were calculated with the regression line and adopted as BPbase. Baseline dopamine D2 receptor BP in healthy volunteers was used, as drug-free D2 BP in the striatum measured with [11C]raclopride in patients with schizophrenia is not significantly different from that in normal controls (Farde et al., 1990; Suhara, 2005).
To determine the key secondary endpoint, the relationship between blonanserin dose and occupancy was analyzed using non-linear regression analysis as described in the Statistical Analysis section.
The other main secondary endpoints were the changes in PANSS total score and CGI-S score from the start of the blonanserin transdermal patch period (visit 2) to the end of the patch treatment period.
Finally, assessments were made regarding patient perception of preparedness to receive blonanserin patch treatment via the Drug Attitude Inventory-10 (DAI-10) at visit 2, visit 4, and the end of the patch treatment period; patient attitudes to patch treatment via a simple questionnaire; and adhesion state of the patches according to a simple 4-step scale.
PET Scan Procedure
An Eminence SET-3000GCT/X (Shimadzu Corporation, Kyoto, Japan) PET scanner was used to measure radioactivity in the striatum. Dynamic PET scanning was performed for 30 to 60 minutes after i.v. bolus injection of 211.1 to 234.6 MBq/1.04 [0.44] (mean [SD]) μg [11C]raclopride. The specific radioactivity of [11C]raclopride was 33.5 to 163.3 GBq/μmol, and the injected mass of [11C]raclopride was 0.47 to 2.35 μg. MRI of the brain was acquired with 1.5T MR imaging, Intera 1.5T Achieva Nova (Philips Medical Systems, Best, the Netherlands) as proton density image (echo time = 17 milliseconds; repetition time = 6000 milliseconds; field of view = 22 cm, 2-dimensional, 256 × 256; slice thickness = 2 mm; number of excitations = 2). These images were used for analysis of the PET scans.
Data Analysis
All emission scans were reconstructed with a Hanning filter cut-off frequency of 0.4. Images were analyzed using PET image analysis software by PMOD version 3.4 (PMOD Technologies Ltd, Zurich, Switzerland). Regions of interest were drawn manually on overlaid co-registered summated PET and MRI images of each patient by PMOD. The average values of right and left regions of interest were used for the analysis. Dopamine D2 receptor binding was quantified using a 3-parameter simplified reference tissue model. The cerebellum was used as reference region because of its negligible dopamine D2 receptor density (Suhara et al., 1999; Tateno et al., 2013).
Safety
Safety was assessed at each visit and at the end of the patch treatment period. The main safety endpoint was the number of patients and cases of adverse events (ie, regardless of causality) and adverse reactions (ie, adverse events considered related to study drug). Adverse events were considered according to seriousness, severity (mild, moderate, severe), outcome (including need for discontinuation), need for treatment, and causal relationship with study drug and were tabulated according to MedDRA/Japanese Version 15.0. Particular attention was given to the number of patients with extrapyramidal adverse events and their frequency. In addition, Drug Induced Extra-Pyramidal Symptoms Scale (DIEPSS) total scores were measured at each visit and at the end of the patch treatment period to determine the change in DIEPSS total score during both blonanserin oral and patch treatment periods. Finally, the number of patients receiving concomitant antiparkinsonian drugs when applying the blonanserin patches was recorded.
Measurements of laboratory values, including serum prolactin levels, in addition to performance of 12-lead ECG were conducted at visits 1, 2, and 4 while vital signs and body weight were measured at each visit and at the end of the patch treatment period.
Statistical Analysis
The pharmacodynamic population for assessment of receptor occupancy and efficacy was defined as patients with at least 1 striatal dopamine D2 receptor occupancy data after application of blonanserin patches, compliance with blonanserin tablets and patches of ≥75%, and who did not take contraindicated antipsychotic or antiparkinsonian agents during the study. The safety analysis population was defined as all patients assigned to blonanserin patch treatment.
Summary statistics were developed according to blonanserin tablet and patch treatment periods for demographic and other baseline characteristics at visit 1 as baseline, dopamine D2 receptor occupancy, change in PANSS and CGI-S scores, safety data (including DIEPSS scores), DAI-10 scores, and adhesion characteristics. Change in scores were estimated from the screening visit (visit 1) for tablet treatment and from the last day of tablet treatment (visit 2) for patch treatment. Further, scatter plots were developed for dopamine D2 receptor occupancy by individual patient data and by dose. The relationship between D2 dopamine receptor occupancy and dose for blonanserin tablets and blonanserin patches was analyzed by non-linear regression analysis according to the equation: occupancy (%) = Bmax × dose / (dose + D50) × 100%, where Bmax is the maximum receptor occupancy and D50 is the dose required to achieve 50% of Bmax. Parameter estimates and their 95% confidence intervals were obtained, but no comparative statistical tests between treatment groups were performed.
The target sample size was originally determined as 20 patients who completed visit 4 (blonanserin patch treatment period), including 5 patients each in the 10-mg/d, 20-mg/d, 40-mg/d, and 60-mg/d blonanserin patch groups.
Results
Patient Disposition and Baseline Characteristics
The disposition of patients throughout the study, including discontinuations, is shown in Figure 2. Of 18 patients who started the blonanserin tablet treatment period, 16 patients were assigned to blonanserin patch treatment (safety analysis set). Of the 16 patients assigned to blonanserin patch treatment, 14 patients completed treatment (pharmacodynamic analysis set). The reason for all discontinuations was recorded as “withdrawal by subject.”
Figure 2.
Disposition of study patients.
The baseline demographic and clinical characteristics of patients in the pharmacodynamic population are shown in Table 1. All groups consisted of men and women, and the age range of patients was 20 to 64 years. Patients who received higher doses of blonanserin (ie, 16 mg/d tablet followed by 40- to 80-mg/d patch groups) generally had more severe disease as evidenced by higher PANSS total and CGI-S scores and had more frequent episodes. Finally, compliance with both oral and patch blonanserin treatment was good (≥75% in all patients).
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Blonanserin transdermal patch group | |||||
|---|---|---|---|---|---|
| Category | 10 mg (n = 3) | 20 mg (n = 2) | 40 mg (n = 3) | 60 mg (n = 3) | 80 mg (n = 3) |
| Sex | |||||
| Male, n (%) | 2 (66.7%) | 1 (50.0%) | 1 (33.3%) | 2 (66.7%) | 2 (66.7%) |
| Female, n (%) | 1 (33.3%) | 1 (50.0%) | 2 (66.7%) | 1 (33.3%) | 1 (33.3%) |
| Age, years, mean (SD) | 51.0 (10.6) | 57.0 (9.9) | 33.3 (10.6) | 51.3 (10.2) | 41.3 (20.6) |
| Height, cm, mean (SD) | 160.0 (8.89) | 161.75 (4.60) | 160.33 (13.80) | 166.0 (9.64) | 165.33 (2.52) |
| Weight, kg, mean (SD) | 60.67 (7.52) | 55.50 (12.73) | 70.50 (15.16) | 81.33 (13.90) | 70.33 (8.96) |
| Schizophrenia subtype (DSM-IV-TR), n (%) | |||||
| Catatonic | 0 | 0 | 1 (33.3%) | 0 | 1 (33.3%) |
| Paranoid | 0 | 1 (50.0%) | 0 | 0 | 2 (66.7%) |
| Residual | 1 (33.3) | 0 | 0 | 1 (33.3%) | 0 |
| Undifferentiated | 2 (66.7) | 1 (50.0%) | 2 (66.7%) | 2 (66.7%) | 0 |
| Duration from first episode to informed consent, mo, mean (SD) | 196.0 (122.3) | 152.5 (212.8) | 86.3 (55.5) | 311.0 (137.9) | 218.7 (257.2) |
| Duration of current episode, mo, mean (SD) | 156.7 (82.6) | 152.5 (212.8) | 83.7 (55.9) | 186.0 (34.2) | 48.7 (62.6) |
| Number of episodes | |||||
| 1 | 1 (33.3%) | 2 (100.0%) | 2 (66.7%) | 0 | 1 (33.3%) |
| 2 | 1 (33.3%) | 0 | 1 (33.3%) | 0 | 1 (33.3%) |
| 3 | 1 (33.3%) | 0 | 0 | 2 (66.7%) | 0 |
| 5 or more | 0 | 0 | 0 | 1 (33.3%) | 1 (33.3%) |
| PANSS total score, mean (SD)a | 69.0 (19.7) | 68.5 (6.4) | 77.0 (9.5) | 80.7 (14.0) | 75.0 (26.0) |
| CGI-S, mean (SD)a | 2.7 (1.5) | 2.5 (0.7) | 3.7 (0.6) | 3.7 (0.6) | 3.3 (0.6) |
Abbreviations: CGI-S; Clinical Global Impressions-Severity of Illness-Severity; PANSS, Positive and Negative Symptom Score.
aBaseline data for PANSS and CGI-S were obtained at visit 2 (baseline of the blonanserin patch period); baseline for all other parameters was the screening visit (Visit 1).
Pharmacodynamic and Efficacy Results
Striatal dopamine D2 receptor occupancy
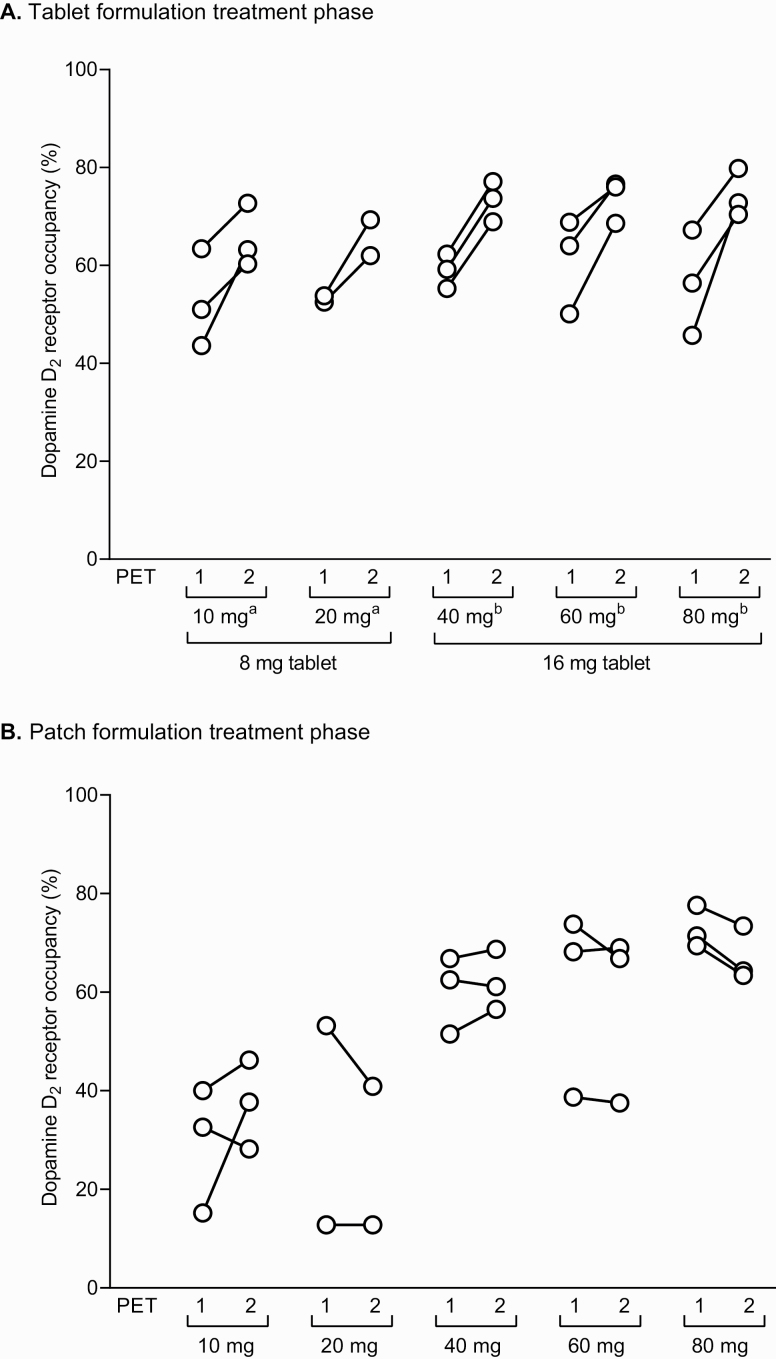
Striatal dopamine D2 receptor occupancy for both the blonanserin tablet and patch treatment periods according to the patch dose assignment groups is shown in Figure 3. During the blonanserin tablet treatment period, blonanserin tablets showed a higher occupancy at the second PET (peak) measurement than at the first PET (trough) measurement. The mean (min, max) occupancy was 59.2% (43.6%, 72.7%) for blonanserin 8 mg/d and 66.3% (45.7%, 79.8%) for blonanserin 16 mg/d. All patients had receptor occupancy in the range of 60%–80% at the peak measurement.
Figure 3.
Striatal dopamine D2 receptor occupancy at first and second PET scans following treatment with blonanserin tablets (A) and blonanserin patches (B) according to patch assignment groups. aThe 10-/20-mg patch groups received tablets at 8 mg/d during oral treatment phase. bThe 40-/60-/80-mg patch groups received tablets at 16 mg/d during oral treatment phase.
During the blonanserin patch treatment period, blonanserin patches showed similar receptor occupancy at the first and second PET measurements (ie, no trough or peak). The mean (min, max) receptor occupancy was 33.3% (15.2%, 46.2%), 29.9% (12.8%, 53.2%), 61.2% (51.5%, 68.7%), 59.0% (37.5%, 73.8%), and 69.9% (63.4%, 77.6%) for 10-mg/d, 20-mg/d, 40-mg/d, 60-mg/d, and 80-mg/d blonanserin patches, respectively. Most or all patients in the 40-mg/d, 60-mg/d, and 80-mg/d groups had receptor occupancy in the range of 60%–80% compared with no patients in either the 10-mg/d or 20-mg/d group.
Comparison of the receptor occupancy for the blonanserin tablet and patch formulations found that mean receptor occupancy (first PET, second PET) with the blonanserin 40-mg/d patch group (60.3%, 62.1%) was within the values for 8-mg/d blonanserin tablet group (52.9%, 65.5%). Similarly, mean receptor occupancy (first PET, second PET) with the 80-mg/d blonanserin patch group (72.8%, 67.0%) was within the values for the 16-mg/d blonanserin tablet group (58.8%, 73.8%).
The relationship between striatal D2 dopamine receptor occupancy and blonanserin dose for blonanserin tablets and patches, as analyzed by the non-linear model described previously, is shown as scatter plots in Figure 4. Further, the mean (SD) receptor occupancy for blonanserin tablets and patches at different doses is shown in Table 2. Dopamine D2 receptor occupancy increased with increasing blonanserin dose for both the tablet and patch formulations. As the 95% confidence interval (CI) of D50 for blonanserin tablets included 0 and the 95% CI of Bmax for blonanserin patches included 100%, additional analysis with Bmax fixed to 100% was conducted. From this analysis, the estimated D50 for blonanserin tablets was 6.9 mg/d, which is slightly below the clinical dose of 8 mg/d, and the estimated D50 for blonanserin patches was 31.9 mg/d (Table 3).
Figure 4.
Relationship between dopamine D2 receptor occupancy and blonanserin dose following treatment with blonanserin tablets (A) or blonanserin patches (B). Dopamine D2 receptor occupancy was plotted against doses and nonlinear regression lines were obtained with the equation of occupancy = Bmax × dose / (D50 + dose). Symbols represent observed occupancy and lines represent regression lines. Dashed lines represent the regression lines with Bmax fixed to 100%.
Table 2.
Summary of Dopamine D2 Receptor Occupancy by Dose
| Dose (mg/d) | Dopamine D2 receptor occupancy (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tablets | Patches | n | Blonanserin tablets | Blonanserin patches | ||||||
| PET 1 (“trough”) | PET 2 (“peak”) | Total | PET 1 | PET 2 | Total | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Min, max | Mean (SD) | Mean (SD) | Mean (SD) | Min, max | |||
| 8 | — | 5 | 52.9 (7.1) | 65.5 (5.3) | 59.2 (8.9) | 43.6, 72.7 | — | — | — | — |
| 16 | — | 9 | 58.8 (7.7) | 73.8 (3.9) | 66.3 (9.7) | 45.7, 79.8 | — | — | — | — |
| 8 | 10 | 3 | 52.7 (10.0) | 65.4 (6.5) | 59.0 (10.3) | 43.6, 72.7 | 29.3 (12.7) | 37.4 (9.0) | 33.3 (10.8) | 15.2, 46.2 |
| 8 | 20 | 2 | 53.2 (0.9) | 65.7 (5.2) | 59.4 (7.8) | 52.5, 69.3 | 33.0 (28.6) | 26.9 (19.9) | 29.9 (20.4) | 12.8, 53.2 |
| 16 | 40 | 3 | 58.9 (3.5) | 73.2 (4.1) | 66.1 (8.6) | 55.3, 77.1 | 60.3 (7.9) | 62.1 (6.2) | 61.2 (6.4) | 51.5, 68.7 |
| 16 | 60 | 3 | 61.0 (9.7) | 73.7 (4.5) | 67.4 (9.7) | 50.1, 76.6 | 60.2 (18.9) | 57.8 (17.6) | 59.0 (16.4) | 37.5, 73.8 |
| 16 | 80 | 3 | 56.4 (10.8) | 74.3 (4.9) | 65.4 (12.3) | 45.7, 79.8 | 72.8 (4.3) | 67.0 (5.5) | 69.9 (5.4) | 63.4, 77.6 |
Abbreviation: PET, positron emission tomography.
Table 3.
Nonlinear Regression Analysis of Dopamine D2 Receptor Occupancy vs Dose
| Bmax (%) | D50 (mg/d) | |||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Tablets | 75.3 | 60.0–90.7 | 2.18 | −0.705–5.06 |
| (n = 14) | 100 | Fixed | 6.90 | 5.68–8.12 |
| Transdermal patches | 85.4 | 63.8–107 | 21.0 | 4.91–37.0 |
| (n = 14) | 100 | Fixed | 31.9 | 24.2–39.5 |
Abbreviations: Bmax, maximum receptor occupancy expected; CI, confidence interval; D50, dose at which 50% of Bmax expected.
Efficacy
At the visit 2 (baseline of blonanserin patch period), PANSS total scores (mean [SD]) ranged between 68.5 (6.4) in the 20-mg/d blonanserin patch group and 80.7 (14.0) in the 60-mg/d blonanserin patch group, whereas CGI-S scores ranged between 2.5 (0.7) in the 20-mg/d blonanserin patch group and 3.7 (0.6) in both the 40-mg/d and 60-mg/d blonanserin patch groups. Neither the PANSS total score nor the CGI-S score worsened in any of the treatment groups from visit 2 to the second week of the transdermal patch application period. Further, there was no relationship observed between PANSS total score or CGI-S and striatal dopamine D2 receptor occupancy during either the oral or transdermal patch treatment periods.
Other Assessment Items
According to the DAI-10, 11 of 16 (68.8%) patients rated blonanserin patch treatment with the same or higher scores than blonanserin tablet treatment. Similarly, results of the patient questionnaire were positive with most patients agreeing that it is better to have a patch formulation available and that patches are easier to continue treatment than tablets. In terms of the adhesive characteristics of the blonanserin transdermal patches, patients overall rated the patch as “mostly attached” 96.7% of the application period (range 91.7%–100.0%). In 1.1% of the application period (range 0.0%–3.6%), patches were rated as between “mostly attached” and “nearly peeled off or peeled off,” and in 2.2% of the application period (range 0.0%–5.6%) it was noted that the patches needed to be reinforced with tape. However, no patients indicated that there was a significant problem with adhesiveness of the blonanserin transdermal patches.
Safety
During the blonanserin tablet treatment period, 5 of 16 patients in the safety population experienced extrapyramidal adverse events, which were considered related to treatment (ie, adverse drug reaction). All 5 patients started concomitant administration of antiparkinsonian agents during tablet days 2 to 6. One patient continued taking antiparkinsonian agents until patch day 6, and others continued administration after completion of patch application. However, there were no serious adverse events or discontinuations during the blonanserin tablet treatment period. During the blonanserin transdermal patch treatment period, 3 adverse events occurred in 2 patients who received 60-mg/d blonanserin patches and 2 adverse events occurred in 2 patients who received 80-mg/d blonanserin patches. A causal relationship with blonanserin could not be ruled out for 1 patient in the 80-mg/d blonanserin patch group who experienced an extrapyramidal-related effect (dyskinesia), which was considered an adverse drug reaction. The other adverse events that occurred in 1 patient each (vertigo/nausea, musculoskeletal pain, burns) were not considered related to blonanserin. Further, there were no serious adverse events or discontinuations during the patch treatment period.
Despite the presence of individual extrapyramidal symptoms, there was no significant change in DIEPSS scores during either treatment period and no association noted between DIEPSS scores and dopamine D2 receptor occupancy. However, concomitant antiparkinsonian agents were administered to 5 patients during both the blonanserin tablet and patch treatment periods, and these were continued after completion of patch application in 4 patients.
Finally, no clinically significant changes in laboratory values, body weight and vital signs, or ECGs were noted during the study.
Discussion
Based on the known therapeutic profile of blonanserin and the findings from a previous receptor occupancy study with the tablet formulation (Tateno et al., 2013), the primary aim of the present study was to investigate the striatal dopamine D2 receptor occupancy associated with daily application of blonanserin transdermal patches. The striatal D2 receptor occupancy 2 weeks after application of blonanserin transdermal patches showed little diurnal variation in peak vs trough levels, which implies the pharmacokinetic and pharmacodynamic rationale underlying blonanserin transdermal patches. The secondary objective of this study was to investigate the relationship between dopamine D2 receptor occupancy and dose for the blonanserin patches alone and compared with blonanserin tablets. In this regard, the extent of D2 receptor occupancy for blonanserin 40-mg/d and 80-mg/d patches were within the trough and peak occupancy for blonanserin 8-mg/d and 16-mg/d tablets, respectively. Further, the half maximal receptor occupancy for tablets and transdermal patches was associated with D50 doses of 6.9 mg/d and 31.9 mg/d, respectively. The fact that the D50 for blonanserin tablets was slightly below the lower approved dose of 8 mg/d suggests that 40 mg/d of blonanserin patches is comparable with blonanserin 8 mg/d tablets, which is slightly higher than the dose associated with the D50 for blonanserin patches.
In general, it is expected that transdermal patch formulations may allow lower effective doses via avoidance of first pass metabolism, compared with oral administration. However, for blonanserin, the expected doses for the patches were much higher than for the tablets. In a separate study, almost 95% of the applied blonanserin was left in the patch after 24-hour application and only 5% of the blonanserin in the patch was absorbed (data not shown). We consider that this explains the inconsistency in the expected dose for the patch formulation.
The peak occupancy of blonanserin tablets in this study was slightly higher than that of blonanserin patches, although satisfactory clinical effects were obtained in a placebo-controlled phase III study and 52-week long-term study of blonanserin patches at doses of 40 mg/d and 80 mg/d (Iwata et al., 2020a, 2020b). Lower peak occupancy in blonanserin patches compared with tablets may be attributed to maintenance of a certain level of both plasma blonanserin concentration and associated dopamine D2 receptor occupancy. In addition, not excessively blocking D2 receptors during the diurnal variation of dose levels may reduce side effects and, in particular, the risk of dopamine hypersensitivity psychosis development, which has been specifically associated with upregulation of dopamine D2 receptors caused by chronic, excessive blockade of D2 receptors by antipsychotics (Nakata et al., 2017).
In relation to other findings of the present study, no changes were seen during the blonanserin patch treatment period in positive or negative symptoms of schizophrenia as assessed by the PANSS total score or the CGI-S. Further, blonanserin patches were associated with a favorable tolerability profile and no excess in adverse events, including extrapyramidal effects, compared with blonanserin tablets. These results are reflected by the lack of change in DIEPSS scores during the study period. Importantly, there were no serious adverse events and no patients discontinued either treatment as a result of adverse events. Based on the present results and previous findings with oral blonanserin, target doses of 40 mg/d and 80 mg/d were established for a phase III study of blonanserin transdermal patches (Iwata et al., 2020b) and a study conducted to properly assess the long-term safety and efficacy of this formulation (Iwata et al., 2020a). Results of the phase III study found statistically significant decreases in the PANSS total score over 6 weeks, especially in the blonanserin 80-mg patch group, which was associated with a moderate effect size (0.555) vs placebo (Iwata et al., 2020b). Both blonanserin 40-mg and 80-mg patches also showed significant improvements compared with placebo for negative symptoms and cognitive disorders in the PANSS 5-factor score. Finally, most patients considered that blonanserin transdermal patches would be a potentially useful option and thought that patches would be at least as easy to continue as tablets. There were also no practical issues with adhesiveness of blonanserin transdermal patches.
The development of a new transdermal route of administration with blonanserin establishes a pharmacokinetic and pharmacodynamic concept that differs from other antipsychotics. Transdermal patches offer a new therapeutic option and can support patients to manage their symptoms in a non-intrusive manner while enabling visual recognition of the application status. A key advantage of patch formulations in the treatment of schizophrenia, in which relapses are often associated with stress and suboptimal therapeutic plasma concentration, is the principle of sustained concentration control in the clinical dose range.
PET scanning has helped overcome limitations of conventional approaches in neuropsychopharmacology research, which are unable to provide an objective index of drug effects or address the possibility that blood drug concentrations may not reflect drug kinetics in target organs (Sekine et al., 2011). Indeed, PET scanning has made it possible to measure the in vivo distribution and kinetics of drugs, correlate drug receptor occupancy in various tissues with therapeutic and adverse effects, and thereby reliably establish doses of antipsychotic agents required for clinical effect and investigation in phase III clinical studies (Arakawa et al., 2008; Sekine et al., 2011; Hargreaves and Rabiner, 2014). The effect of blonanserin tablets on dopamine receptor occupancy has been investigated previously in trials using PET scanning (Tateno et al., 2013, 2018). Similar to the present study, these studies found that dopamine D2 receptor occupancy occupied the range of 60%–80% with blonanserin tablet doses of 8 mg/d to 16 mg/d, such that the range of such favorable peak occupancy was well matched with the clinical dose. In this respect, the current study exemplifies well 1 aspect of the recognized translational potential of PET imaging in allowing the determination and refinement of dose ranges for investigation in clinical studies (Hargreaves and Rabiner, 2014).
Strengths and Limitations
The main strength of this study is the use of PET scanning as an objective measure that has been repeatedly shown to allow correlation between dopamine D2 receptor occupancy and optimal dose. In turn, the ability to antagonize central dopamine D2 receptors has been linked to the therapeutic efficacy of antipsychotics. In contrast, the main limitations of this study are the small sample size, which did not reach the specified targets, and the brief duration of treatment periods. However, in terms of the first limitation, the study population is similar in size to that of other studies using PET scanning, which provides a more objective measure of therapeutic action and thus helps mitigate the effects of potential bias. The brief duration of treatment may have reduced the ability of this study to properly determine the relative adherence benefits of the blonanserin transdermal patch formulation. Studies of adherence are conducted over periods of at least 12 weeks or considerably longer in the case of observational studies. In this regard, in a 52-week study, DAI-10 scores and questionnaire results indicated that the positive attitude toward blonanserin transdermal patch treatment held by a certain proportion of patients did not change during long-term treatment (Iwata et al., 2020a). There is also a methodological limitation of using base dopamine D2 occupancy in healthy volunteers to calculate the dopamine D2 receptor occupancy, similar to that noted in a previous study (Tateno et al., 2013). A limitation concerning dosing relates to the fact that blonanserin tablets can be administered at doses up to 24 mg/d, but there is no information whether patients who take blonanserin tablets at doses higher than 16 mg/d would have the same effect as those administered blonanserin patches at 80 mg/d. Further investigation of this dosing issue is needed. Finally, the lack of reference to the concentration of blonanserin is a potential limitation of this study. However, the objective of this study was to estimate the dosage of blonanserin patch for use in a phase III study, and this could be achieved through the relationship between dose and dopamine D2 occupancy.
Conclusions
Blonanserin transdermal patches represent a potential new option for patients with schizophrenia who require ongoing antipsychotic treatment. The results of the current study, which implemented PET imaging as a useful tool for dose setting of blonanserin patches, support the pharmacokinetic characteristics of blonanserin patches and confirm their lower diurnal variability compared with blonanserin tablets. Blonanserin patch doses of 40 mg/d or 80 mg/d were selected as the doses to be verified in a phase III study, which subsequently did verify the safety and efficacy of these doses. These results are of practical relevance to clinical studies of blonanserin transdermal patches, especially in relation to dosing.
Acknowledgments
The authors thank all patients, investigators, and staff who participated in this study. The authors also thank Yoshiko Okamoto, PhD, and Mark Snape, MB BS, CMPP, of inScience Communications, Springer Healthcare, who provided medical writing support.
This work was supported by Sumitomo Dainippon Pharma Co., Ltd, Tokyo, Japan.
Statement of Interest
Hironori Nishibe and Hiroyoshi Kakuyama are employees of Sumitomo Dainippon Pharma Co., Ltd, Tokyo, Japan. All other authors declare no conflict of interest relevant to the submitted manuscript.
References
- Arakawa R, Ito H, Takano A, Takahashi H, Morimoto T, Sassa T, Ohta K, Kato M, Okubo Y, Suhara T (2008) Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 197:229–235. [DOI] [PubMed] [Google Scholar]
- Baba S, Enomoto T, Horisawa T, Hashimoto T, Ono M (2015) Blonanserin extensively occupies rat dopamine D3 receptors at antipsychotic dose range. J Pharmacol Sci 127:326–331. [DOI] [PubMed] [Google Scholar]
- Citrome L, Zeni CM, Correll CU (2019) Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry 80:18n–r12554.. [DOI] [PubMed] [Google Scholar]
- Deeks ED, Keating GM (2010) Blonanserin: a review of its use in the management of schizophrenia. CNS Drugs 24:65–84. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Halldin C, Sedvall G (1988) Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 45:71–76. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordström AL, Hall H, Sedvall G (1990) D2 dopamine receptors in neuroleptic-naive schizophrenic patients. A positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry 47:213–219. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordström AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S, Halldin C (1995) Variability in D2-dopamine receptor density and affinity: a PET study with [11C]raclopride in man. Synapse 20:200–208. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Rabiner EA (2014) Translational PET imaging research. Neurobiol Dis 61:32–38. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Nakamura H, Murasaki M (2019) Blonanserin versus haloperidol in Japanese patients with schizophrenia: a phase 3, 8-week, double-blind, multicenter, randomized controlled study. Neuropsychopharmacol Rep 39:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Okubo Y, Halldin C, Farde L (1999) Mapping of central D2 dopamine receptors in man using [11C]raclopride: PET with anatomic standardization technique. Neuroimage 9:235–242. [DOI] [PubMed] [Google Scholar]
- Iwata N, Ishigooka J, Naoi I, Matsumoto M, Kanamori Y, Nakamura H, Higuchi T (2020a) Long-term safety and efficacy of blonanserin transdermal patches in Japanese patients with schizophrenia: a 52-week open-label, multicenter study. CNS Drugs 34:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Ishigooka J, Kim WH, Yoon BH, Lin SK, Sulaiman AH, Cosca R, Wang L, Suchkov Y, Agarkov A, Watabe K, Matsui T, Sato T, Inoue Y, Higuchi T, Correll CU, Kane JM (2020b) Efficacy and safety of blonanserin transdermal patch in patients with schizophrenia: a 6-week randomized, double-blind, placebo-controlled, multicenter study. Schizophr Res 215:408–415. [DOI] [PubMed] [Google Scholar]
- Kinoshita T (2008) Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to determine safety and effectiveness in schizophrenic patients (Japan-wide study). Jpn J Clin Psychopharmacol 11:135–153. [Google Scholar]
- Kishi T, Matsui Y, Matsuda Y, Katsuki A, Hori H, Yanagimoto H, Sanada K, Morita K, Yoshimura R, Shoji Y, Hagi K, Iwata N (2019) Efficacy, tolerability, and safety of blonanserin in schizophrenia: an updated and extended systematic review and meta-analysis of randomized controlled trials. Pharmacopsychiatry 52:52–62. [DOI] [PubMed] [Google Scholar]
- Li H, Yao C, Shi J, Yang F, Qi S, Wang L, Zhang H, Li J, Wang C, Wang C, Liu C, Li L, Wang Q, Li K, Luo X, Gu N (2015) Comparative study of the efficacy and safety between blonanserin and risperidone for the treatment of schizophrenia in Chinese patients: a double-blind, parallel-group multicenter randomized trial. J Psychiatr Res 69:102–109. [DOI] [PubMed] [Google Scholar]
- Mohr P, Volavka J (2012) Adherence and long-acting injectable antipsychotics in schizophrenia: an update. J Psychiatry Neurol Sci 25:285–296. [Google Scholar]
- Murasaki M (2007) Long-term clinical study of blonanserin for schizophrenia: a multicenter open study to determine safety and effectiveness in schizophrenic patients (Kanagawa Region Clinical Psychopharmacology Study Group). Jpn J Clin Psychopharmacol 10:2241–2257. [Google Scholar]
- Nakata Y, Kanahara N, Iyo M (2017) Dopamine supersensitivity psychosis in schizophrenia: concepts and implications in clinical practice. J Psychopharmacol 31:1511–1518. [DOI] [PubMed] [Google Scholar]
- Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G (1993) Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33:227–235. [DOI] [PubMed] [Google Scholar]
- Sekine M, Maeda J, Shimada H, Nogami T, Arakawa R, Takano H, Higuchi M, Ito H, Okubo Y, Suhara T (2011) Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci 9:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, Okubo Y, Nakashima Y, Ito H, TanadaS,HalldinC,Farde L (1999) Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol 2:73–82. [DOI] [PubMed] [Google Scholar]
- Suhara T, Okubo Y, Yasuno F, Sudo Y, Inoue M, Ichimiya T, Nakashima Y, Nakayama K, Tanada S, Suzuki K, Halldin C, Farde L (2005). Decreased dopamine D2 receptor binding in the anterior cingulated cortex in schizophrenia. Arch Gen Psychiatry 59:25–30. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Suzuki M, Uchiyama M (2013) One-year follow-up study of psychotic patients treated with blonanserin: a case series. Asia Pac Psychiatry 5:164–167. [DOI] [PubMed] [Google Scholar]
- Tateno A, Arakawa R, Okumura M, Fukuta H, Honjo K, Ishihara K, Nakamura H, Kumita S, Okubo Y (2013) Striatal and extrastriatal dopamine D2 receptor occupancy by a novel antipsychotic, blonanserin: a PET study with [11C]raclopride and [11C]FLB 457 in schizophrenia. J Clin Psychopharmacol 33:162–169. [DOI] [PubMed] [Google Scholar]
- Tateno A, Sakayori T, Kim WC, Honjo K, Nakayama H, Arakawa R, Okubo Y (2018) Comparison of dopamine D3 and D2 receptor occupancies by a single dose of blonanserin in healthy subjects: a positron emission tomography study with [11C]-(+)-PHNO. Int J Neuropsychopharmacol 21:522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T, Kurumiya S (2007) Pharmacological profile of blonanserin. Jpn J Clin Psychopharmacol 10:1263–1272. [Google Scholar]
- Yilmaz Z, Zai CC, Hwang R, Mann S, Arenovich T, Remington G, Daskalakis ZJ (2012) Antipsychotics, dopamine D(2) receptor occupancy and clinical improvement in schizophrenia: a meta-analysis. Schizophr Res 140:214–220. [DOI] [PubMed] [Google Scholar]