FIGURE 3.
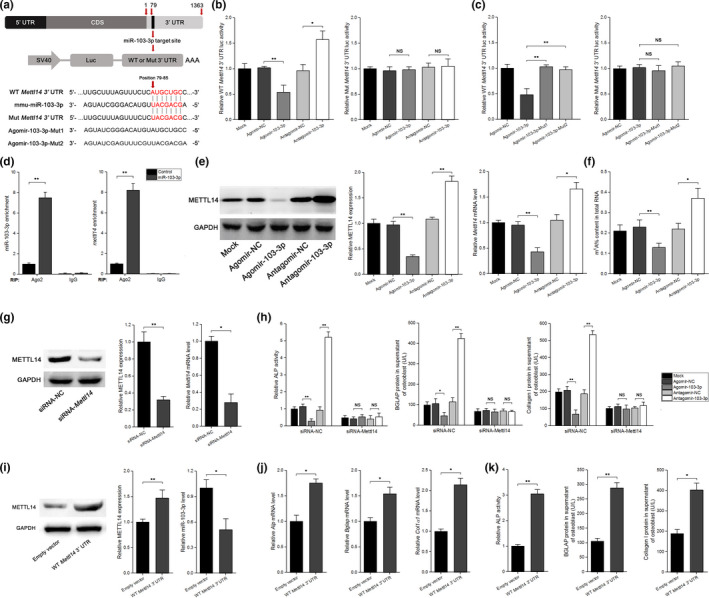
miR‐103‐3p directly targets Mettl14 to inhibit the function of osteoblasts in vitro. (a) Schematic illustration of the design of luciferase reporters containing the WT Mettl14 3′UTR or the site‐directed mutant Mettl14 3’UTR. The pairing regions of miR‐103‐3p and the sequences of the two Agomir‐103‐3p mutants (Agomir‐103‐3p‐Mut1 and Agomir‐103‐3p‐Mut2) are also shown. (b) The effects of agomir‐103‐3p, antagomir‐103‐3p or the corresponding negative controls (agomir‐NC and antagomir‐NC) on luciferase activity in MC3 T3‐E1 cells transfected with either the WT Mettl14 3′UTR reporter (left) or the mutant Mettl14 3′UTR reporter (right) (n = 3). (c) The effect of agomir‐NC, agomir‐103‐3p, and mutated agomir‐103‐3p on luciferase activity in MC3 T3‐E1 cells transfected with either the WT Mettl14 3′UTR reporter (left) or the mutant Mettl14 3’UTR reporter (right) (n = 3). (d) RNA immunoprecipitation (RIP) assays showing the association with Ago2 of both miR‐103‐3p (left) and Mettl14 mRNA (right) in MC3 T3‐E1 cells overexpressing miR‐103‐3p (n = 3). (e) The effect of agomir‐103‐3p, antagomir‐103‐3p, or the corresponding negative controls on the METTL14 protein (left and middle) and Mettl14 mRNA (right) levels in primary mouse osteoblasts (n = 4). (f) The effect of agomir‐103‐3p, antagomir‐103‐3p, or the corresponding negative controls on the m6A content in total RNA (n = 3). (g) Western blot (left and middle) and real‐time PCR (right) analysis of the knockdown efficiency of Mettl14‐siRNA after treatment with a specific siRNA targeting Mettl14 (siRNA‐Mettl14) or a nonspecific siRNA control (siRNA‐NC) (n = 3). (h) ALP activity (left) and the amount of BGLAP protein (middle) and collagen I (right) in the supernatant of primary mouse osteoblasts after silencing of Mettl14 with siRNA‐Mettl14 and treatment with agomir‐103‐3p, antagomir‐103‐3p, or the corresponding negative controls (n = 3). The results from siRNA‐NC and mock transfection are also shown. (i) Western blot (left and middle) and real‐time PCR (right) analysis of the effect of WT Mettl14 3′UTR on the METTL14 protein (left and middle) and miR‐103‐3p (right) levels in primary mouse osteoblasts (n = 3). (j) Real‐time PCR analysis of the changes in the mRNA levels of the osteoblast differentiation marker genes Alp (left), Bglap (middle), and Col1α1 (right) in primary mouse osteoblasts after blockade of miR‐103‐3p binding to Mettl14 via overexpression of WT Mettl14 3′UTR (n = 3). (k) ALP activity (left) and the amount of BGLAP protein (middle) and collagen I (right) in the supernatant of primary mouse osteoblasts after blockade of miR‐103‐3p binding to Mettl14 via overexpression of WT Mettl14 3′UTR (n = 3). The results from the Empty vector and mock transfection are also shown. All data are the mean ± SD. *p < 0.05, **p < 0.01. One‐way ANOVA with a post hoc test was performed, and the significance of differences between two groups was determined with Student's t test
