FIGURE 4.
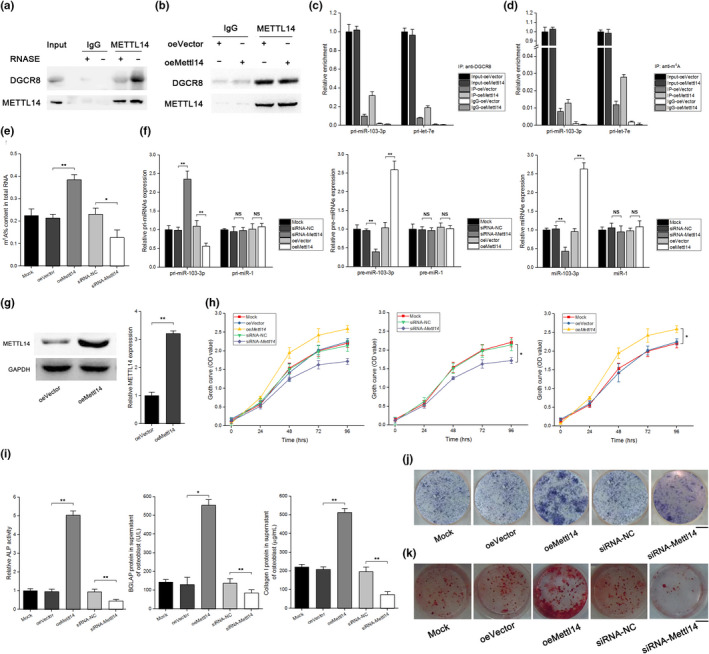
METTL14‐dependent m6A methylation regulates miR‐103‐3p processing by the microprocessor protein DGCR8 and modulates osteoblast activity in vitro. (a) Coimmunoprecipitation (IP) of the METTL14‐interacting protein DGCR8. Western blotting with anti‐DGCR8 and anti‐METTL14 antibodies and immunoglobulin G (IgG) antibody was used as a control for IP. (b) IP of DGCR8, METTL14, and associated RNA from control MC3 T3‐E1 cells or METTL14‐overexpressing MC3 T3‐E1 cells. The cells were UV‐cross‐linked before IP. Western blotting or immunoblotting was conducted using the antibodies described above. (c) Real‐time PCR analysis of pri‐miR‐103‐3p binding to DGCR8 in IP assay of DGCR8‐associated RNA from control and METTL14‐overexpressing MC3 T3‐E1 cells (n = 3). Pri‐let‐7e was used as a positive control. (d) Real‐time PCR analysis of the pri‐miR‐103‐3p m6A modification level determined by IP of m6A‐modified miRNA in control or METTL14‐overexpressing MC3 T3‐E1 cells (n = 3). Pri‐let‐7e was used as a positive control. (e) The effect of oeMettl14, siRNA‐Mettl14, or the corresponding negative controls on the m6A content in total RNA (n = 3). (f) Real‐time PCR analysis of pri‐miR‐103‐3p (left), pre‐miR‐103‐3p (middle), and miR‐103‐3p (right) in METTL14 knockdown and overexpression MC3 T3‐E1 cells (n = 3). (g) Western blot analysis of the overexpression efficiency of Mettl14 plasmid after treatment of primary mouse osteoblasts with a specific Mettl14 plasmid (oeMettl14) or an empty vector (n = 3). (h) WST‐8 assay of changes in primary mouse osteoblast growth at 24–96 h after treatment with oeMettl14, siRNA‐Mettl14 or the corresponding negative controls (n = 3). (i) ALP activity (left) and the amount of BALP protein (middle) and collagen I (right) in the supernatant of primary mouse osteoblasts after treatment with oeMettl14, siRNA‐Mettl14 or the corresponding negative controls (n = 3). (j) Representative images of ALP staining of primary mouse osteoblasts after treatment with oeMettl14, siRNA‐Mettl14, or the corresponding negative controls (n = 3). Scale bar, 10 mm. (k) Alizarin red staining of calcium deposition in primary mouse osteoblasts after treatment with oeMettl14, siRNA‐Mettl14, or the corresponding negative controls in osteogenic medium for 21 days. Scale bar, 10 mm. All data are the mean ± SD. *p < 0.05, **p < 0.01. One‐way ANOVA with a post hoc test was performed, and the significance of differences between two groups was determined with Student's t test
