Figure 2.
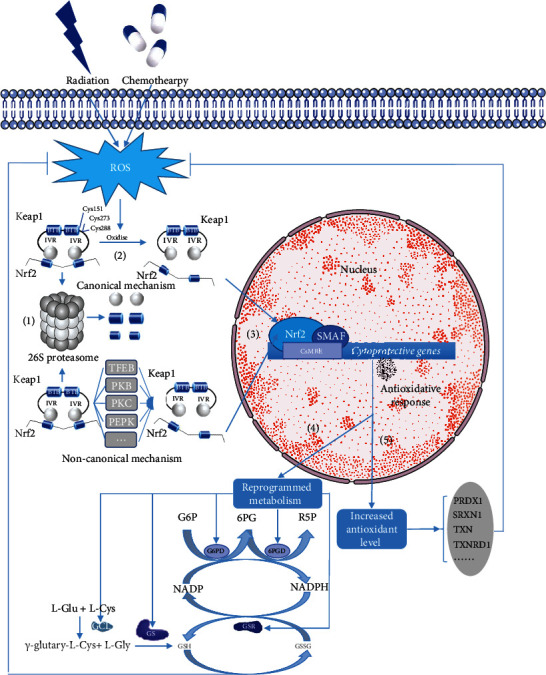
The role of the Keap1-Nrf2 signalling pathway in antioxidative stress-related radio- and chemoresistance. (1) Degradation of Nrf2. Under normal conditions, Keap1 combines with Nrf2 to promote the degradation of Nrf2 by the 26S proteasome. (2) Activation of Nrf2. Radiation and chemotherapies increase the effective ROS concentration in cancer cells via the redox reaction of water and direct production of ROS, respectively. Later, increased ROS oxidise three cysteines within Keap1 to activate Nrf2 by dissociating Nrf2 from Keap1 and slowing down the speed of Nfr2 degradation. (3) Translocation of Nrf2. Activated Nrf2 translocates into the nucleus, forming the Nrf2-sMAF heterodimer with one of the small MAF (sMAF) proteins. The Nrf2-sMAF heterodimer binds to the CNC-sMAF binding element (CsMBE), resulting in increased antioxidant levels and reprogrammed metabolism. (4) Increased antioxidant enzymes. Various antioxidant genes including peroxiredoxin 1 (PRDX1), sulfiredoxin 1 (SRXN1), thioredoxin (TXN), and thioredoxin reductase 1 (TXNRD1) can be activated by Nrf2. (5) Reprogrammed metabolism. G6PD and 6PGD are the two major enzymes in the pentose phosphate pathway (PPP), which generates NADPH. Glutamate cysteine ligase (GCL) and glutathione synthase (GS) are the two rate-limiting enzymes in glutathione (GSH) de novo synthesis. Nrf2 promotes the translation and expression of GCL, GS, GSR, G6PD, and 6PGD. Increased antioxidant enzymes and reprogrammed metabolism can protect cancer cells from ROS-triggered cell death, leading to radio- and chemoresistance.
