Figure 3.
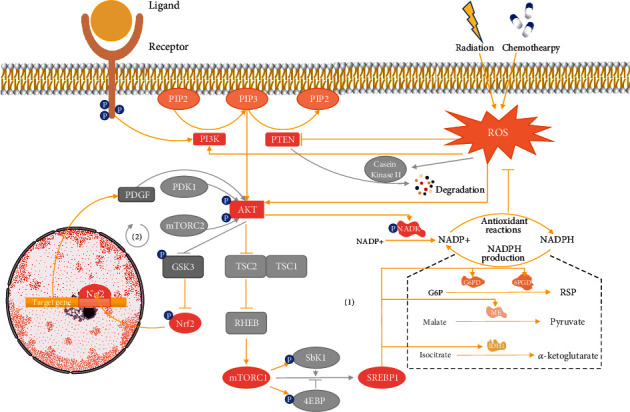
(1) The role of the PI3K-AKT signalling pathway in antioxidative stress related to radio- and chemoresistance. Phosphoinositide-3-kinases (PI3Ks) phosphorylate phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), which subsequently recruits and activates protein kinase B (PKB/AKT). However, these processes can be inhibited by phosphatase and tensin homolog (PTEN), which dephosphorylates PIP3 to PIP2. As radiation and chemotherapy continuously generate ROS via the redox reaction of water and direct production of ROS, respectively, increased intracellular ROS activate the PI3K-AKT pathway by directly promoting PI3K and AKT and inhibiting PTEN. PI3K-AKT signalling serves as defence against ROS by promoting NADPH production. Activated AKT regulates NADPH metabolism in direct and indirect ways via NAD+ kinase (NADK) and rapamycin complex 1 (mTORC1), respectively. NADK is the unique cytosolic enzyme that catalyses the phosphorylation of NAD+ to NADP+, enlarging the size of the NADP+ and NADPH pool. Downstream of mTORC1, sterol regulatory element-binding protein (SREBP) stimulates the expression of G6PD, 6PGD, IDH1, and ME, reducing NADP+ to NADPH. (2) The loop formed between AKT and Nrf2. Glycogen synthase kinase 3 (GSK3) and platelet-derived growth factor (PDGF) act as a linker to connect AKT with Nrf2. PI3K-AKT signalling inhibits the Keap1-independent degradation of Nrf2 by phosphorylating GSK3. Activated Nrf2 translocates to the nucleus and upregulates the transcription of PDGF, binding with its cognate receptors to stimulate AKT.
