Fig. 2.
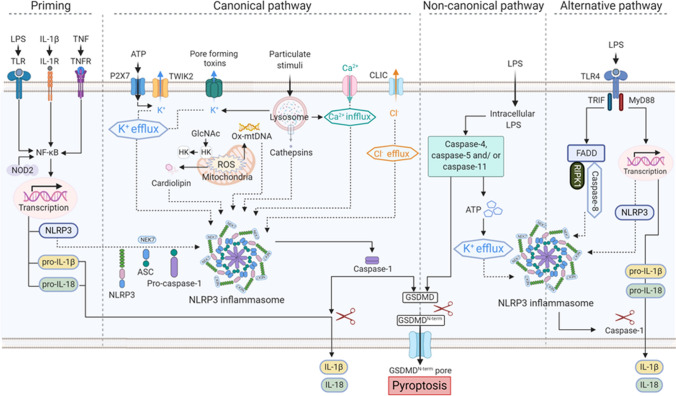
Schematic illustration of the NLRP3 inflammasome pathway. Priming is the first step of NLRP3 inflammasome activation. Priming is induced by Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-containing protein-2 (NOD2). Priming can also be induced by cytokines, such as tumor necrosis factor (TNF) and interleukin 1 beta (IL-1β), which activate nuclear factor-κB (NF-κB) and gene transcription. Priming upregulates the expression of inflammasome components, such as NLRP3, caspase-1, and pro-IL-1β. The second step is an activation signal mediated by numerous PAMPs, DAMPs, fungi, bacteria producing pore-forming toxins, viruses, and environmental irritants. These activation signals include the efflux of potassium (K+) and chloride ions (Cl−), the influx of calcium ions (Ca2+), lysosomal disruption, mitochondrial dysfunction, metabolic changes, and trans-Golgi disassembly. Decomposition of the bacterial cell wall component peptidoglycan during bacterial infection releases N-acetylglucosamine (GlcNAc) in the lysosomes. Hexokinase (HK), which is located on the mitochondrial membrane, binds GlcNAc. GlcNAc-induced hexokinase relocalization promotes NLRP3 inflammasome activation regardless of K+ efflux. Mitochondrial dysfunction and the release of mtROS and mitochondrial DNA (mtDNA) into the cytosol are the major upstream events in NLRP3 activation. NEK7, a regulator of the NLRP3 inflammasome, interacts with NLRP3 through the catalytic domain of NEK7 and the LRR domain of NLRP3. Activation of the inflammasome causes caspase-1 activation resulting in the maturation and release of IL-1β/IL-18 and pyroptosis. Caspase-1 cleaves and releases gasdermin D (GSDMD). The amino terminal cell death domain of GSDMD (GSDMDNterm) inserts into the membrane to form the pores and induce pyroptosis. Noncanonical NLRP3 inflammasome activation is induced by gram-negative bacteria. Intracellular LPS delivered to the cytosol via infection activates human caspase-4/-5 and mouse caspase-11. Human caspase-4/-5 and mouse caspase-11 indirectly induce the activation of pro-IL-1β or pro-IL-18 by promoting NLRP3 inflammasome activation. Activated caspase-4, -5, and -11 cleave GSDMD and cause pyroptosis. The alternative inflammasome pathway is induced by TRIF-RIPK1-FADD-caspase-8 signaling that does not require K+ efflux and does not induce pyroptosis