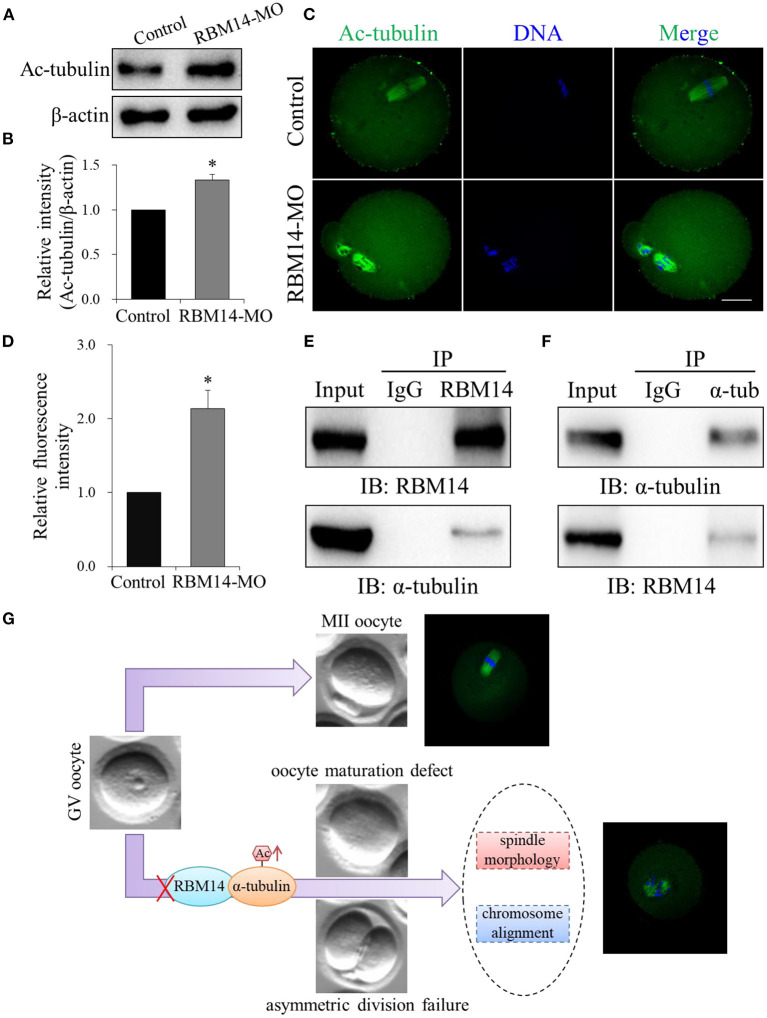
Figure 5.
RBM14 depletion increased α-tubulin acetylation in mouse oocytes by interacting with α-tubulin. (A) Western blot analysis showing that the level of α-tubulin acetylation was increased in RBM14-MO injected oocytes. (B) Acetylated α-tubulin band intensities normalized to β-actin. (C) Representative confocal images of control and RBM14-depleted oocytes. Green = ac-tubulin antibody, blue = DNA (Hoechst 33342). Scale bar = 20 μm. (D) Relative fluorescence intensities of acetylated α-tubulin in control and RBM14-depleted oocytes. (E) Co-IP was performed with whole cell extracts from NIH/3T3 cells to determine the interaction between RBM14 and α-tubulin. Protein lysate was incubated with RBM14 antibody or nonspecific rabbit IgG antibody, followed by Western blot analysis using an α-tubulin antibody. (F) Reciprocal co-IP in NIH/3T3 whole cell lysates using α-tubulin antibody or nonspecific rabbit IgG antibody to pull down α-tubulin, followed by Western blot analysis using a RBM14 antibody. (G) A schematic model showing the potential pathway that RBM14 is involved in oocyte meiotic maturation. Data are mean ± SEM of three independent experiments. *P < 0.05.