Abstract
Purpose
Does IDEF mapping help monitor the technical process of IUI and explore the potential improvements which might contribute to increased pregnancy and live birth rates?
Method
Retrospective analysis of 1729 homologous IUI cycles of couples attending a fertility clinic in a university hospital setting. Standardized conventional semen parameters were analyzed and the semen samples prepared via discontinuous density gradient centrifugation.
Results
There was no significant association between sperm concentration, motility and morphology (analysis phase), and pregnancy outcome. Only female and male ages were significantly associated with the pregnancy outcome. There was a significant difference in the odds on clinical pregnancies and live births when analysis was ≤ 21 min initiated, and < 107 min between sample production and IUI, adjusted for male and female age.
Conclusions
Adjusting for the couple’s age, we could show that time intervals between semen production and analysis and IUI when kept low significantly influenced clinical pregnancies and live births.
Keywords: IDEF modeling, Intrauterine insemination, Semen parameters, Density gradient centrifugation
Introduction
Intrauterine insemination (IUI) is implemented on a large scale worldwide as a simple non-invasive first-line therapy of assisted reproduction. But, overall success rates per IUI cycle are rather low in comparison with in vitro fertilization/intra-cytoplasmic sperm injection (IVF/ICSI), with a generally accepted range of 10–20% clinical pregnancy rate per cycle for all etiologies [1, 2].
Several factors have been proposed to influence the likelihood of pregnancy after IUI. These factors may be related to the couple (clinical determinants), such as infertility diagnosis, duration of infertility, age, ovarian stimulation protocols, number of follicles, induction of ovulation, day of insemination, number of inseminations, and endometrial thickness [3–10], or ejaculate related (laboratory determinants), such as semen analysis parameters, semen processing, processing time, threshold recovery, inseminating concentration and volume, and insemination catheters [6, 9–15].
Cohlen et al. have reviewed and assessed the most recent evidence on IUI concluding that most of the presented “evidence” is of moderate or (very) low quality [16]. Consequently, the authors drafted recommendations on why and how to perform IUI treatments. However, out of the 13 draft recommendations, 11 dealt with the female clinical aspects and only 2 covered the male part—semen quality and semen preparation technique.
Combining various clinical and laboratory determinants that influence the likelihood of pregnancy makes it difficult to study the impact of one on the other. Another factor which makes the review of literature difficult on this topic is a lack of standardization of the technical procedure required for IUI, resulting in interlaboratory variations in pregnancy rates.
The world data (ICMART - International Committee for Monitoring Assisted Reproductive Technology, 2011) on the global IUI pregnancy rate (PR) was 12.0% and delivery rate (DR) 8.0% per cycle [17] with a wide range among different countries reporting 8.5–21.7% PR and 1.3–17.9% DR, respectively.
The IUI procedure, according to Lemmens et al., can roughly be separated into three steps: diagnosis and indication, cycle preparation, and the technical stage [18]. The last step, including the whole process between semen collection and insemination, is described by the WHO laboratory manual [19]. The significance of these guidelines has been further substantiated by Bjorndahl et al. [20]. The Vienna Consensus report [21] between ESHRE (European Society of Human Reproduction and Embryology) Special Interest Group Embryology (SIGE) and Alpha Scientists in Reproductive Medicine has even established appropriate laboratory performance indicators for sperm preparation for assisted reproduction. But these guidelines do not cover every step of the IUI sample evaluation, preparation, and insemination. Before establishing best practice guidance on the clinical level within the field of infertility, it is of prime importance to standardize the technical/laboratory aspect first. When monitoring such a technical process, relevant qualifiers, confounders, and endpoints should be identified to evaluate the laboratory contribution to patient care. In search for a functional modeling methodology, we implemented Integration Definition (IDEF) developed by the US Air Force (IEEE-Institute of Electrical and Electronics Engineers Standards Association 1998) to understand how the laboratory activities around a homologous IUI cycle relate to one another and at the same time an attempt was made to identify and document the core laboratory activities during sample preparation leading to increased pregnancy and live birth rates.
Materials and methods
Study population
Men presenting at the Centre for Reproductive Medicine, Antwerp University Hospital, Belgium, for infertility diagnosis and treatment via IUI between April 2013 and March 2018 were included in this study. The project was approved by the Institutional Ethical Committee. In total, 2205 homologous IUI cycles were included in this study. Male exclusion criteria were < 2 and > 7 days of abstinence, use of cryopreserved semen, use of two consecutive semen samples for IUI, and semen preparation not via density gradient centrifugation. In Belgium, IUI reimbursement takes into account the female age (until 43 years of age) but not the male age. Consequently, female aged > 43 years were also excluded. In total, 662 couples were included undergoing 1729 IUI cycles (mean: 2.6 cycles/couple, SD = 1.7, range 1–13 cycles).
Ovarian stimulation/cycle monitoring
IUI was performed in spontaneous cycles or cycles stimulated with clomifene citrate or gonadotrophins in a low dose. When 1 or 2 dominant follicles were present, IUI was planned 34–38 h after triggering with hCG or 24–28 h after detection of spontaneous LH surge. When three or more dominant follicles were present, a follicle reduction to 1 or 2 was executed or the cycle was canceled according to the patients’ preference. Follicle reduction was done under vaginal ultrasound guidance after administration of an oral analgesic as paracetamol and performed before hCG administration. This occurred in a minor percentage of stimulated cycles (< 5%) and did not affect outcome.
Semen analysis
Patients were instructed to maintain 2–7 days of sexual abstinence. All semen samples were collected at the laboratory and any ejaculate fraction missing was reported. Samples were weighed and analysis was initiated within 60 min after ejaculation—including sperm concentration using an improved Neubauer hemocytometer (Marienfeld GmbH, Lauda-Königshofen, Germany) combined with a positive displacement pipette (Microman, Gilson Inc., Middleton, WI, USA); sperm motility including progressive and total motile sperms; and sperm morphology adapting the modified Papanicolaou stain (Sigma-Aldrich Inc., St. Louis, MO, USA).
Sperm processing
A part of the same semen sample was treated with a two-step discontinuous density gradient [22] using PureSperm® (Nidacon, International AB, Gothenburg, Sweden). Briefly, 40% and 80% PureSperm® density gradient were prepared using 1.5 ml of each suspension. Semen (1.0–1.5 ml) was layered on the top of each gradient and centrifuged for 20 min at 300 g. After which, the upper layer seminal plasma, the 40% upper layer, and the 40–80% interface were discarded and the remaining spermatozoa in the 80% pellet were collected from the bottom of the tube and washed once, for 10 min, with human tubal fluid (HTF Hepes, Gynotec, Malden, The Netherlands) supplemented with albumin (human albumin 20%, CAF-DCF, Brussels, Belgium).
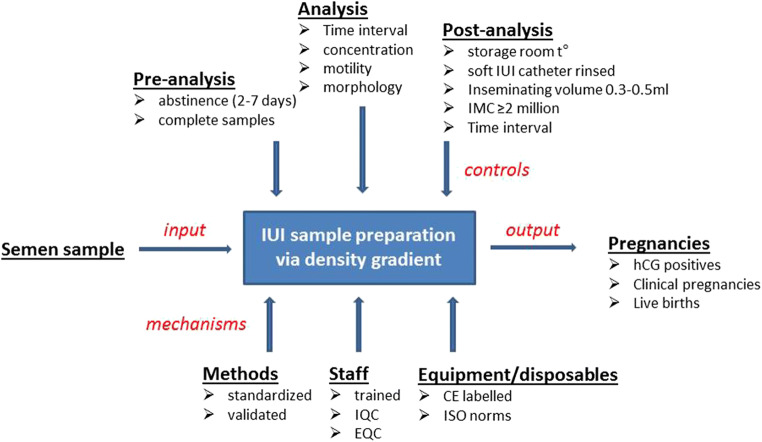
IDEF modeling
In an IDEF model, the central box represents a single process/activity. Arrows signifying inputs, controls, outputs, and mechanisms are added to the box. Inputs get transformed or changed in some way in order to create the outputs. Controls direct the activity in the process and mechanisms are the resources required to complete the process. This modeling [23] provided a context for the processes and activities involving laboratory determinants for IUI.
Semen sample is the “input” which gets evaluated and prepared by density gradient centrifugation before being inseminated in the uterine cavity to give pregnancies as the “output” (Fig. 1). Mechanisms (resources and tools required to complete the process) which might influence the technical process of IUI sample evaluation and preparation were held under control. These were at the levels of methodology—for which only standardized methods were chosen (WHO 2010 recommendations) but validated before implementation, and complying with the checklist for acceptability reported by Björndahl et al. for both semen parameters and semen preparation [20]. Semen preparation via density gradient centrifugation was previously validated under standardized conditions to provide the optimal inseminating motile sperm count [24], an important predictive parameter for IUI success. Another mechanism influencing the process was at the laboratory staff level. All staff members who performed the analysis were trained in basic semen analysis via ESHRE - Basic Semen Analysis Courses [25, 26], and participated regularly in internal and external quality control programs (Institute of Public Health, Belgium and ESHRE External Quality Control Schemes, Sweden) [27] for both semen parameters and density gradient centrifugation. Laboratory equipment (centrifuges, improved Neubauer hemocytometers, air and positive displacement pipettes, heated plates, and laboratory counters) and disposables (slides, coverslips, and pipette tips) conform to the international standards of ISO 15189 (International Standards Organization, 2012). This left us with the opportunity to see which analytical part of semen sample analysis/preparation would control or limit the whole process.
Fig. 1.
IDEF–IUI activity model (ejaculate determinants). In IDEF, a box describes the function to be mapped. Arrows signifying inputs, controls, outputs, and mechanisms are added to the box. Inputs get transformed/changed to create the outputs. Controls guide, facilitate, or constrain the process. Mechanisms are the resources and tools that are required to complete the process. IQC/EQC, internal/external quality controls; IMC, inseminating progressive motile count
The technical aspect (controls facilitating or constraining the process) was subdivided into three parts: pre-analysis, analysis, and post-analysis.
For the pre-analysis phase, only complete samples were included with 2–7 days of abstinence as suggested by the WHO (2010). During analysis, pre-wash concentration, motility, and morphology were determined. After semen preparation, during the post-analysis phase, the sample was prepared and kept at room temperature until insemination. Before insemination, the motility and the concentration were checked and the total inseminating motile count (IMC) of ≥ 2 M progressive spermatozoa, a yield of an absolute lower limit sufficient to contemplate IUI [24], was inseminated using a soft IUI catheter (Wallace® Intrauterine Insemination Catheters, Cooper Surgical, The Hague, The Netherlands) rinsed with HTF and albumin. The inseminating volume was held constant between 0.3 and 0.5 ml.
Statistical analysis
Statistical analyses were conducted using SAS version 9.4 and R version 3.6.2. Data are summarized with estimated means and standard errors (M ± SE) using a linear mixed model with the semen parameter as outcome and pregnancy (positive hCG) as predictor. Couple was added as a random intercept. Values were back transformed after logarithmic transformations where necessary. P values of pregnancy are reported. To see which of the measured semen parameters were associated with pregnancy (positive hCG), a generalized linear mixed effects model was fitted with pregnancy as binary outcome and the semen parameter as predictor. Here also, the couple was included as a random intercept. These associations were also studied in a model with addition of female and male age as a covariate (one age variable at the time).
Independent cycle data was obtained by including a random cycle per couple which was only used to construct a receiver operating characteristic (ROC) curve relating time between semen production and start analysis and time between semen production and IUI, respectively, to pregnancy (positive hCG). Threshold criteria were determined using the Youden J index. The time variables were then made binary using this threshold and their association with the different pregnancy outcomes (positive hCG, clinical pregnancy, and live birth) was considered using a generalized linear mixed effects model as before in the full data set. Statistical significance was set at P value < 0.05.
Results
Pregnancy outcome
Mean female age included was 31.7 years (SD = 4.5, range 21.6–42.99) and male age was 34.4 (SD = 5.8, range 22.7–56.6) years. Of the 1729 cycles included, 254 (14.7%) resulted in a positive hCG. Of these, 13 were biochemical (0.8%) and 241 (13.9%) clinical pregnancies. Of the clinical pregnancies, 28 (11.6%) had a spontaneous abortion and in another two abortion was induced (0.8%). Two pregnancies were ectopic (0.8%), one was immature (0.4%), and 2 were stillborn (0.8%), resulting finally in 206 live births (11.9%).
In 1710/1729 cycles, hCG was administered (98.9%) and only in 19 cycles (1.1%) a spontaneous LH surge was observed. Three pregnancies were obtained with LH surge (15.8%) and 251 with hCG administration (14.7%) but with no significance (P > 0.439). In our center, we define an LH surge as LH > 30 IU/L and only then we carry out IUI the day after.
Semen parameters and IUI outcome
The estimated means and SE of all semen parameters in the pregnant (hCG positive) and non-pregnant groups together with the P value for pregnancy are summarized in Table 1. The table also reports values of significance and OR (95% CI) of the semen parameters in the generalized linear mixed effects model, unadjusted and adjusted for male age. P values with inclusion of female age were similar (not reported). There was no significant association between sperm concentration, motility and morphology (analysis phase), and pregnancy outcome. Only female and male ages are significantly associated with the pregnancy outcome. For male age, the odds ratio on pregnancy is 0.958 (95% CI [0.930, 0.987]), meaning that an increase of 1 year of age decreases odds on pregnancy with 4%. The same is seen for female age where the odds ratio is 0.961 (95% CI [0.927, 0.997]).
Table 1.
Estimates and P values of the linear mixed model (semen parameters as outcome) and the generalized linear mixed effects (GLME) model (pregnancy as outcome)
| Semen parameters | Linear mixed model | Unadjusted GLME model | Adjusted GLME model for male age | |||||
|---|---|---|---|---|---|---|---|---|
| Pregnant group, 254 cycles, 228 couples | Non-pregnant group, 1475 cycles, 575 couples | Total group, 1729 cycles, 662 couples | P value pregnancy | OR semen parameter | P value | OR semen parameter | P value | |
| Abstinence (days) | 3.6 (0.09) | 3.6 (0.05) | 3.6 (0.04) | 0.599 | 0.994 [0.893,1.105] | 0.907 | 1.002 [0.900,1.115] | 0.973 |
| Volume (ml)* | 3.8 (0.10) | 3.7 (0.07) | 3.8 (0.07) | 0.446 | 1.044 [0.978,1.114] | 0.197 | 1.042 [0.977,1.112] | 0.210 |
| Sperm concentration (M/ml)* | 42.2 (2.1) | 42.3 (1.5) | 42.2 (1.4) | 0.991 | 1.000 [0.997,1.003] | 0.976 | 1.000 [0.997,1.003] | 0.931 |
| Total sperm count (M/ejaculate)* | 163.8 (8.2) | 158.0 (5.2) | 159.0 (5.0) | 0.458 | 1.000 [1.000,1.001] | 0.514 | 1.000 [1.000,1.001] | 0.516 |
| Progressive sperm motility (%) | 48.6 (0.71) | 47.7 (0.45) | 47.9 (0.43) | 0.185 | 1.005 [0.993,1.018] | 0.406 | 1.004 [0.992,1.017] | 0.521 |
| Total sperm motility (%) | 55.1 (0.78) | 54.6 (0.46) | 54.7 (0.43) | 0.538 | 1.003 [0.992,1.015] | 0.587 | 1.002 [0.991,1.014] | 0.738 |
| Total motile sperm count (M/ejaculate)* | 87.2 (4.9) | 83.1 (3.1) | 83.9 (3.0) | 0.371 | 1.000 [0.999,1.001] | 0.542 | 1.000 [0.999,1.001] | 0.595 |
| Sperm morphology (%) | 6.5 (0.21) | 6.6 (0.16) | 6.6 (0.16) | 0.371 | 0.977 [0.941,1.014] | 0.225 | 0.980 [0.944,1.017] | 0.289 |
| Inseminating progressive motile count (M)* | 5.7 (0.29) | 5.6 (0.18) | 5.6 (0.17) | 0.920 | 0.986 [0.960,1.014] | 0.323 | 0.984 [0.957,1.012] | 0.257 |
| Time interval between semen production and start analysis* | 28.9 (0.49) | 30.1 (0.25) | 29.9 (0.23) | 0.037 | 0.985 [0.969,1.002] | 0.090 | 0.987 [0.970,1.003] | 0.119 |
| Time between semen production and IUI* | 102.0 (1.55) | 103.9 (0.74) | 103.6 (0.68) | 0.250 | 0.996 [0.990,1.002] | 0.212 | 0.997 [0.991,1.003] | 0.275 |
| Female age (years) | 31.9 (0.18) | 31.9 (0.17) | 31.9 (0.17) | 0.344 | 0.961 [0.927,0.997] | 0.034 | ||
| Male age (years) | 34.5 (0.23) | 34.5 (0.23) | 34.5 (0.23) | 0.341 | 0.958 [0.930,0.987] | 0.005 | ||
Values are estimated means (SE) or OR (odds ratio [95% CI])
*Means and SE back transformed after logarithmic transformation in the linear mixed model
Analysis and post-analysis time intervals
As the data are clustered within couple, a random cycle per couple was taken to look at the Youden threshold for the ROC curve using time intervals to predict pregnancy. For the time between semen production and start analysis, a threshold of 21 min was found (specificity = 459/516 = 89.0% and sensitivity = 26/139 = 18.7%; AUC = 0.523). For the time between semen production and IUI, a threshold of 107 min gave the maximum Youden index (specificity = 198/490 = 40.4% and sensitivity = 91/133 = 68.4%; AUC = 0.519).
These thresholds were then used to make the time variables binary for the full data set (1729 cycles). A generalized linear mixed effects model with the binary time variable as predictor was fitted to see if the binary variables are significantly associated with the different outcomes. For all outcomes, P values and odds ratios (95% CI) for the different pregnancy outcomes for time below threshold versus time above threshold are given in Table 2. An adjusted model with male age included was also fitted and reported (results for female age were similar and not reported). All time variables are significantly associated resulting in a doubling of the odds on the pregnancy outcome if the time between semen production and start analysis is 21 min or smaller, and 1.6 times the odds if the time between semen production and IUI is 107 min or smaller. We also looked into the models where the time between semen production and IUI was involved and added hCG administration versus spontaneous LH surge to the model. This variable was in none of the models significant (P > 0.439 in all cases) and this had no impact on the estimated OR or confidence intervals.
Table 2.
P values and odds ratio (95% CI) for binary time variables in a generalized linear mixed effects model, unadjusted and adjusted for male age
| Pregnancy outcome | Time intervals between (min) | OR time unadjusted | P value time unadjusted | OR time adjusted for male age | P value time adjusted for male age |
|---|---|---|---|---|---|
| Positive hCG | Semen production and start analysis, ≤ 21 | 1.96 [1.32,2.91] | 0.0009 | 1.93 [1.30,2.86] | 0.0011 |
| Semen production and IUI ≤ 107 | 1.53 [1.11,2.12] | 0.0093 | 1.52 [1.10,2.09] | 0.0114 | |
| Clinical pregnancy | Semen production and start analysis, ≤ 21 | 1.98 [1.32,2.99] | 0.0010 | 1.95 [1.30,2.94] | 0.0013 |
| Semen production and IUI ≤ 107 | 1.56 [1.12,2.19] | 0.0089 | 1.55 [1.11,2.17] | 0.0108 | |
| Live birth | Semen production and start analysis, ≤ 21 | 2.17 [1.39,3.41] | 0.0007 | 2.14 [1.37,3.34] | 0.0009 |
| Semen production and IUI ≤ 107 | 1.63 [1.11,2.38] | 0.0118 | 1.61 [1.10,2.35] | 0.0137 |
Discussion
For the first time, IDEF mapping was used to monitor the technical process of IUI and to explore the potential improvements which might contribute to patient care. This model does not predict the probability of pregnancy after IUI but signifies the inevitable need to adhere to strict guidelines as a step towards standardization.
Despite the extensive literature on IUI, there is great controversy in the effectiveness of the IUI procedure. Lack of standardization during semen analysis and the wide variations in methods adopted during sperm preparation make the laboratory procedure a confounding factor by itself when interpreting these data [28, 29].
In a systematic review, Ombelet et al. suggest that inseminating motile count between 0.8 and 5 million, sperm morphology using strict criteria > 4%, total motile count of 5–10 million, and total motility of > 30% in the neat semen have a substantial discriminative performance in an IUI program [29]. Although these parameters can predict failure (high specificity), a poor sensitivity for predicting pregnancy was observed. Mankus et al. investigated the relationship between TMC and live births, and found that ROC analysis yielded no correlation concluding that pre-wash TMC is not predictive of live births in IUI cycles [30]. Lemmens et al. reported that sperm morphology, TMC, and number of inseminated progressively motile spermatozoa were not predictive for ongoing pregnancy after the first IUI cycle [31]. Our study confirms these observations for all semen parameters in the neat semen and progressive motile count inseminated. Although inseminating progressive motile count was independently validated and the threshold used to ensure high chances for pregnancies [24], there was no significant difference in clinical pregnancies (P = 0.9208, OR = 1.029, 95% CI [0.584, 1.813]) and live births (P = 0.9155, OR = 0.967, 95% CI [0.521, 1.794]) between patients receiving ≥ 2 million or not.
Although IUI (with partner/donor sperm) is reimbursed until the age of 43 years, our results support multiple studies [8, 9, 32–35] that have negatively linked female age with IUI success. On the other hand, effect of paternal age on IUI outcomes has been controversial and poorly explored. Belloc et al. reported a significant decrease in pregnancies in men older than 45 years [36], as compared to those younger than 30 years (9.3% vs. 12.3%, respectively). Univariate statistical analysis revealed male age as a covariate significantly influencing clinical pregnancy rate per cycle [37], although this could not be substantiated by multivariate generalized estimating equation. On the other hand, Tatsumi et al. showed that advanced paternal age had no adverse effect on pregnancy rates in couples undergoing IUI, and that pregnancy outcomes depended on female age alone [38]. The observed negative effect of male age, in our study, agrees well with that observed in terms of live births after IVF/ICSI, namely 4.1% decrease in the odds with each year of increase in male age [39]. We have assessed the effects of male and female age with semen variables that could impact negatively the success rate of IUI. To account for repeated IUI cycles within couples, all regression analyses were carried out using couples as a random intercept, which allowed taking into account the effects of within-couple variability. In this way, the independent impact of male and female age on clinical outcome was studied while minimizing the effect of confounding factors. The influence of male age on pregnancy outcome was obviously not via descriptive semen parameters. We have previously shown [24] that sperm DNA damage has been implicated in both male age and infertility.
Reprotoxicity of equipment and disposables is an important problem concerning laboratory procedures. Safety and effectivity were tested before release using sperm survival assay as described in [40]. Moreover, the soft IUI catheter was rinsed with culture medium to remove any impurities before use as is already the case for embryo transfer catheter [41].
Regarding time intervals, there is controversy in literature. Yavas and Selub found that semen collection in the clinic led to a shorter time interval between sample production and analysis (< 26 min) resulting in higher pregnancy rates [42]. This is in line with our criteria of ≤ 21 min for analysis time interval. There was a significant difference in the odds on clinical pregnancies and live births when analysis was ≤ 21 min, adjusted for male and female age. But, human ejaculates collected for in vitro procedures show rapid increases in osmolality. Although spermatozoa adjust to this high osmolality, motility becomes negatively and irreversibly affected when exposed to a medium with a lower osmolality during semen preparation [43]. This might be a contributing factor to the results obtained in this study.
In a larger study population, the group of Song observed no impact of time interval on IUI pregnancy [44]. While, Fauque et al. observed that the time interval from the end of sperm preparation to IUI had a potential positive impact on pregnancy rate if held between 40 and 80 min [45]. However, the authors do not mention the total time interval between semen production and IUI. Although multicentric, the study was hampered by a lack of standardization. The authors denoted that performing IUI too soon after sperm preparation (< 40 min) might reduce the success rate due to reduced decondensation of sperm chromatin [45]. On the other hand, increased time interval after sperm preparation (> 80 min) may be negatively influenced by in vitro conditions increasing sperm DNA fragmentation [46]. More than 98% of the samples in our study were produced in the clinic. As far as the post-analysis time interval is concerned, between sample production and IUI, a similar trend was observed, when adjusted for male and female age. There was a statistical significant difference in odds on clinical pregnancies and live births when the time factor was lower than 107 min, in contrast to Yavas and Selub, who observed higher pregnancy rates when this time interval was held < 90 min [42]. Nevertheless, the majority of the reports propose to avoid long time intervals both before and after analysis.
For the first time, a model is used to help standardize the IUI laboratory procedure. When mechanisms and the pre-analysis phase were held under control, semen parameters did not influence IUI outcome. However, under standardized laboratory conditions, male and female age independently affected IUI outcomes. Adjusting for the couple’s age, we could show that time intervals between semen production and analysis and IUI should be kept low. Nevertheless, a number of potential confounding factors that were not possible to include in the study, such as extreme male factor, parity, and ovarian stimulation, may have played a role in our findings. A good registration of all technical variables is inevitable. This paves the way to look at the prognostic values of patient (male and female) characteristics, subsequently developing guidelines on both clinical and technical levels. This laboratory standardization can help set up multicentric protocols to answer the questions still unanswered regarding IUI.
Acknowledgments
The authors acknowledge Luc Delbeke (past Medical Director, Centre for Reproductive Medicine) and Tina Schiltz, Christine Croes, Els Smet, and Zehra Kara for preparing the semen samples.
Authors’ contribution
U.P. substantially contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content, and final approval of the version to be published. D.D.N. substantially contributed to the conception, acquisition of data, interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published. E.R. was involved in statistical analysis and interpretation of data, revising it critically for important intellectual content, and final approval of the version to be published. All the other authors conducted additional data acquisition, critical revision of the article, and approved the final version of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ombelet W, Puttemans P, Bosmans E. Intrauterine insemination: a first-step procedure in the algorithm of male subfertility treatment. Hum Reprod. 1995;10 Suppl 1:90–102. doi: 10.1093/humrep/10.suppl_1.90. [DOI] [PubMed] [Google Scholar]
- 2.Duran HE, Morshedi M, Kruger T, Oehninger S. Intrauterine insemination: a systematic review on determinants of success. Hum Reprod Update. 2002;8:373–384. doi: 10.1093/humupd/8.4.373. [DOI] [PubMed] [Google Scholar]
- 3.Goverde AJ, McDonnell J, Vermeiden JP, Schats R, Rutten FF, Schoemaker J. Intrauterine insemination or in-vitro fertilisation in idiopathic subfertility and male subfertility: a randomised trial and cost-effectiveness analysis. Lancet. 2000;355:13–18. doi: 10.1016/S0140-6736(99)04002-7. [DOI] [PubMed] [Google Scholar]
- 4.Cohlen BJ, te Velde ER, van Kooij RJ, Looman CW, Habbema JD. Controlled ovarian hyperstimulation and intrauterine insemination for treating male subfertility: a controlled study. Hum Reprod. 1998;13:1553–1558. doi: 10.1093/humrep/13.6.1553. [DOI] [PubMed] [Google Scholar]
- 5.Khalil MR, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. Intrauterine insemination with donor semen. An evaluation of prognostic factors based on a review of 1131 cycles. Acta Obstet Gynecol Scand. 2001;80:342–348. doi: 10.1034/j.1600-0412.2001.080004342.x. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson MJ, Amissah-Arthur JB, Thompson KA, Kasraie JL, Bentick B. Prognostic indicators for intrauterine insemination (IUI): statistical model for IUI success. Hum Reprod. 1996;11:1892–1896. doi: 10.1093/oxfordjournals.humrep.a019513. [DOI] [PubMed] [Google Scholar]
- 7.Ragni G, Maggioni P, Guermandi E, Testa A, Baroni E, Colombo M, Crosignani PG. Efficacy of double intrauterine insemination in controlled ovarian hyperstimulation cycles. Fertil Steril. 1999;72:619–622. doi: 10.1016/s0015-0282(99)00326-x. [DOI] [PubMed] [Google Scholar]
- 8.Badawy A, Elnashar A, Eltotongy M. Effect of sperm morphology and number on success of intrauterine insemination. Fertil Steril. 2009;91:777–781. doi: 10.1016/j.fertnstert.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Montanaro Gauci M, Kruger TF, Coetzee K, Smith K, Van Der Merwe JP, Lombard CJ. Stepwise regression analysis to study male and female factors impacting on pregnancy rate in an intrauterine insemination programme. Andrologia. 2001;33:135–141. doi: 10.1046/j.1439-0272.2001.00428.x. [DOI] [PubMed] [Google Scholar]
- 10.Yavuz A, Demirci O, Sozen H, Uludogan M. Predictive factors influencing pregnancy rates after intrauterine insemination. Iran J Reprod Med. 2013;11:227–234. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Vlahos N, Wyncott D, Petrella C, Garcia J, Zacur H, Wallach EE. Impact of semen characteristics on the success of intrauterine insemination. J Assist Reprod Genet. 2004;21:143–148. doi: 10.1023/B:JARG.0000031246.76666.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ombelet W, Vandeput H, Van de Putte G, Cox A, Janssen M, Jacobs P, et al. Intrauterine insemination after ovarian stimulation with clomiphene citrate: predictive potential of inseminating motile count and sperm morphology. Hum Reprod. 1997;12:1458–1463. doi: 10.1093/humrep/12.7.1458. [DOI] [PubMed] [Google Scholar]
- 13.Carrell DT, Kuneck PH, Peterson CM, Hatasaka HH, Jones KP, Campbell BF. A randomized, prospective analysis of five sperm preparation techniques before intrauterine insemination of husband sperm. Fertil Steril. 1998;69:122–126. doi: 10.1016/s0015-0282(97)00446-9. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Li B, Fan LQ, Zhu WB, Chen XJ, Feng JH, Yang CL, Zhang YH. Does sperm morphology affect the outcome of intrauterine insemination in patients with normal sperm concentration and motility? Andrologia. 2012;44:299–304. doi: 10.1111/j.1439-0272.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 15.van Weert JM, Repping S, Van Voorhis BJ, van der Veen F, Bossuyt PM, Mol BW. Performance of the postwash total motile sperm count as a predictor of pregnancy at the time of intrauterine insemination: a meta-analysis. Fertil Steril. 2004;82:612–620. doi: 10.1016/j.fertnstert.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Cohlen B, Bijkerk A, Van der Poel S, Ombelet W. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update. 2018;24:300–319. doi: 10.1093/humupd/dmx041. [DOI] [PubMed] [Google Scholar]
- 17.Adamson GD, de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2011. Fertil Steril. 2018;110:1067–1080. doi: 10.1016/j.fertnstert.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 18.Lemmens L, Kos S, Beijer C, Braat DDM, Nelen W, Wetzels AMM. Techniques used for IUI: is it time for a change? Hum Reprod. 2017;32:1835–1845. doi: 10.1093/humrep/dex223. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization, WHO Laboratory manual for the examination and processing of human semen. 5th ed. Cambridge; 2010.
- 20.Bjorndahl L, Barratt CL, Mortimer D, Jouannet P. How to count sperm properly’: checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–232. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
- 21.The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod BioMed Online. 2017;35:494–510. [DOI] [PubMed]
- 22.Punjabi U, Gerris J, van Bijlen J, Delbeke L, Gielis M, Buytaert P. Comparison between different pre-treatment techniques for sperm recovery prior to intrauterine insemination, GIFT or IVF. Hum Reprod. 1990;5:75–83. doi: 10.1093/oxfordjournals.humrep.a137046. [DOI] [PubMed] [Google Scholar]
- 23.Mortimer D, Mortimer S. Process and systems. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 24.Punjabi U, De Neubourg D, Van Mulders H, Cassauwers W, Peeters K. Validating semen processing for an intrauterine program should take into consideration the inputs, actions and the outputs of the process. Andrologia. 2018;e12977. [DOI] [PubMed]
- 25.Punjabi U, Spiessens C. Basic semen analysis courses: experience in Belgium. In: Ombelet WBE, Vandeput H, Vereecken A, Renier M, Hoomans E, editors. Modern ART in the 2000’s - andrology in the nineties. London: Parthenon Publishing Group; 1998. pp. 107–113. [Google Scholar]
- 26.Bjorndahl L, Barratt CL, Fraser LR, Kvist U, Mortimer D. ESHRE basic semen analysis courses 1995–1999: immediate beneficial effects of standardized training. Hum Reprod. 2002;17:1299–1305. doi: 10.1093/humrep/17.5.1299. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi U, Wyns C, Mahmoud A, Vernelen K, China B, Verheyen G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology. 2016;4:1084–1093. doi: 10.1111/andr.12230. [DOI] [PubMed] [Google Scholar]
- 28.Van Waart J, Kruger TF, Lombard CJ, Ombelet W. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7:495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 29.Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T. Semen quality and prediction of IUI success in male subfertility: a systematic review. Reprod BioMed Online. 2014;28:300–309. doi: 10.1016/j.rbmo.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Mankus EB, Holden AE, Seeker PM, Kampschmidt JC, McLaughlin JE, Schenken RS, et al. Prewash total motile count is a poor predictor of live birth in intrauterine insemination cycles. Fertil Steril. 2019;111:708–713. doi: 10.1016/j.fertnstert.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemmens L, Kos S, Beijer C, Brinkman JW, van der Horst FA, van den Hoven L, et al. Predictive value of sperm morphology and progressively motile sperm count for pregnancy outcomes in intrauterine insemination. Fertil Steril. 2016;105:1462–1468. doi: 10.1016/j.fertnstert.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Campana A, Sakkas D, Stalberg A, Bianchi PG, Comte I, Pache T, et al. Intrauterine insemination: evaluation of the results according to the woman’s age, sperm quality, total sperm count per insemination and life table analysis. Hum Reprod. 1996;11:732–736. doi: 10.1093/oxfordjournals.humrep.a019244. [DOI] [PubMed] [Google Scholar]
- 33.Stone BA, Vargyas JM, Ringler GE, Stein AL, Marrs RP. Determinants of the outcome of intrauterine insemination: analysis of outcomes of 9963 consecutive cycles. Am J Obstet Gynecol. 1999;180:1522–1534. doi: 10.1016/s0002-9378(99)70048-7. [DOI] [PubMed] [Google Scholar]
- 34.Hendin BN, Falcone T, Hallak J, Nelson DR, Vemullapalli S, Goldberg J, Thomas Jr AJ, Agarwal A. The effect of patient and semen characteristics on live birth rates following intrauterine insemination: a retrospective study. J Assist Reprod Genet. 2000;17:245–252. doi: 10.1023/A:1009402214820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinelli L, Courbière B, Achard V, Jouve E, Deveze C, Gnisci A, Grillo JM, Paulmyer-Lacroix O. Prognosis factors of pregnancy after intrauterine insemination with the husband’s sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril. 2014;101:994–1000. doi: 10.1016/j.fertnstert.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Belloc S, Cohen-Bacrie P, Benkhalifa M, Cohen-Bacrie M, De Mouzon J, Hazout A, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod BioMed Online. 2008;17:392–397. doi: 10.1016/s1472-6483(10)60223-4. [DOI] [PubMed] [Google Scholar]
- 37.Thijssen A, Creemers A, Van der Elst W, Creemers E, Vandormael E, Dhont N, et al. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: a prospective cohort study. Reprod BioMed Online. 2017;34:463–472. doi: 10.1016/j.rbmo.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Tatsumi T, Ishida E, Tatsumi K, Okada Y, Saito T, Kubota T, Saito H. Advanced paternal age alone does not adversely affect pregnancy or live-birth rates or sperm parameters following intrauterine insemination. Reprod Med Biol. 2018;17:459–465. doi: 10.1002/rmb2.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horta F, Vollenhoven B, Healey M, Busija L, Catt S, Temple-Smith P. Male ageing is negatively associated with the chance of live birth in IVF/ICSI cycles for idiopathic infertility. Hum Reprod. 2019;34:2523–2532. doi: 10.1093/humrep/dez223. [DOI] [PubMed] [Google Scholar]
- 40.Nijs M, Franssen K, Cox A, Wissmann D, Ruis H, Ombelet W. Reprotoxicity of intrauterine insemination and in vitro fertilization-embryo transfer disposables and products: a 4-year survey. Fertil Steril. 2009;92:527–535. doi: 10.1016/j.fertnstert.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Pont JC, Patrat C, Fauque P, Camp ML, Gayet V, Wolf JP. Pre-washing catheter dramatically improves the post intrauterine insemination pregnancy rate. Gynecol Obstet Fertil. 2012;40:356–359. doi: 10.1016/j.gyobfe.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Yavas Y, Selub MR. Intrauterine insemination (IUI) pregnancy outcome is enhanced by shorter intervals from semen collection to sperm wash, from sperm wash to IUI time, and from semen collection to IUI time. Fertil Steril. 2004;82:1638–1647. doi: 10.1016/j.fertnstert.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 43.Holmes E, Björndahl L, Kvist U. Hypotonic challenge reduces human sperm motility through coiling and folding of the tail. Andrologia. 2020;00:e13859. doi: 10.1111/and.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song GJ, Herko R, Lewis V. Location of semen collection and time interval from collection to use for intrauterine insemination. Fertil Steril. 2007;88:1689–1691. doi: 10.1016/j.fertnstert.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 45.Fauque P, Lehert P, Lamotte M, Bettahar-Lebugle K, Bailly A, Diligent C, et al. Clinical success of intrauterine insemination cycles is affected by the sperm preparation time. Fertil Steril. 2014;101:1618–23.e1–3. doi: 10.1016/j.fertnstert.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Hammadeh ME, Strehler E, Zeginiadou T, Rosenbaum P, Schmidt W. Chromatin decondensation of human sperm in vitro and its relation to fertilization rate after ICSI. Arch Androl. 2001;47:83–87. doi: 10.1080/014850101316901262. [DOI] [PubMed] [Google Scholar]