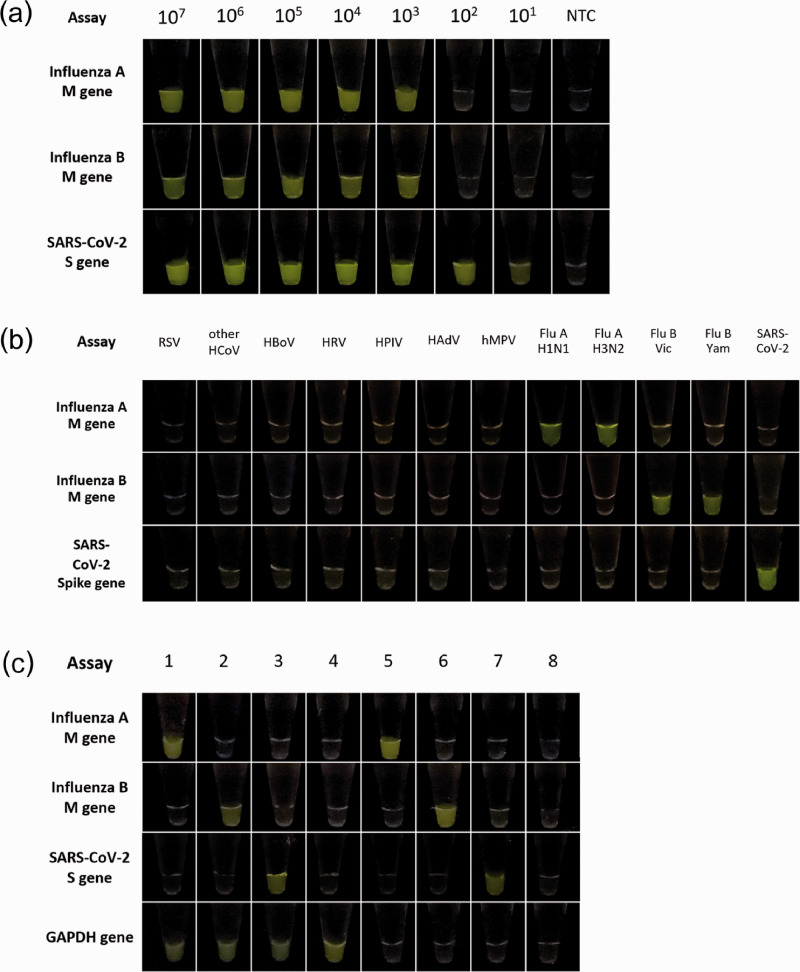
Figure 2.
Detection of SARS-CoV-2, influenza A virus, and influenza B virus based on CRISPR-Cas12a. (a) Limit of detection for influenza A virus, influenza B virus, and SARS-CoV-2 based on RT-RPA and CRISPR-Cas12a assay. NTC: no template control. (b) Cross-reactivities testing of RT-RPA and CRISPR-Cas12a assay against several respiratory viruses. RSV: respiratory syncytial virus; HCoV: human coronavirus strains OC43 and NL63; HBoV: human bocavirus; HRV: human rhinovirus; HPIV: human parainfluenza virus; HAdV: human adenovirus; hMPV: human metapneumovirus; Flu A-H1N1: influenza A virus subtype pH1N1; Flu A-H3N2: influenza A virus subtype H3N2; Flu B-Vic: influenza B virus Victoria lineage; Flu B-Yam: influenza B virus Yamagata lineage and SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. (c) Representative results of RT-RPA and CRISPR-Cas12a assay for IAV, IBV, SARS-CoV-2, and GAPDH by testing with RNA extracted from clinical samples. Samples 1 and 5 were as IAV-positive samples; samples 2 and 6 were IBV-positive samples; samples 3 and 7 were SARS-CoV-2-positive samples; sample 4 was a negative sample; sample 8 was interpreted as inadequate specimen collection. (A color version of this figure is available in the online journal.)