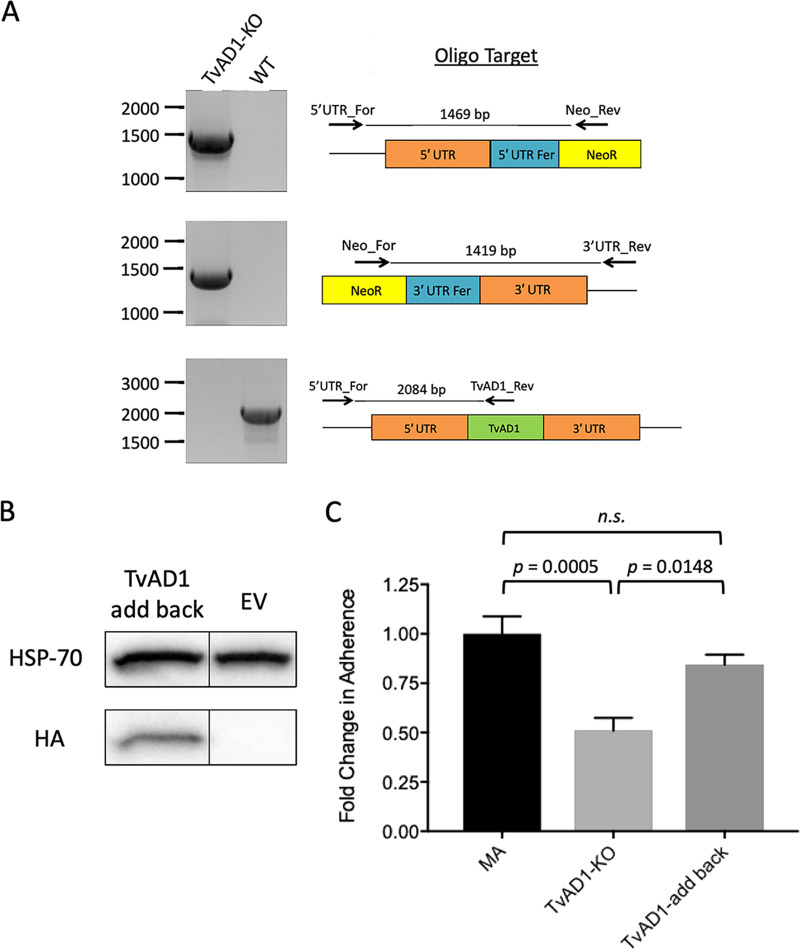
FIG 4.
CRISPR-Cas9 knockout of TvAD1 significantly reduces adherence of MA parasites to BPH-1 cell monolayers. (A) PCR analysis of the TvAD1 knockout (TvAD1-KO) and wild-type (WT) parasites for the presence of the neo gene in the TvAD1 locus. PCRs using primers to amplify the 5′ UTR integration site (5UTR_For + Neo_Rev) yielded the expected 1,469-bp product (top panel), while primers used to amplify the 3′ UTR integration (Neo_For + 3UTR_Rev) yielded the expected 1,419-bp product (middle panel), showing that the neomycin gene is present in the TvAD1 locus. The intact TvAD1 locus product of 2,084 bp detected in WT parasites using primers located upstream of the 5′ integration site and within the TvAD1 gene (5UTR_For + 157internal_Rev (bottom panel) is absent in TvAD1-KO parasites. (B) Episomal expression of a C-terminal 2× HA-tagged TvAD1 protein in the KO background (TvAD1-add back) was confirmed by anti-HA immunoblot analyses and compared with parasites transfected with empty vector (EV). Hsp-70 was used as a loading control. Expected sizes for Hsp-70 and TvAD1-add back are ∼70 kDa and ∼37 kDa, respectively. The black line between TvAD1-add back and EV indicates the blot was spliced to remove a lane between the samples. (C) Attachment of TvAD1-KO and TvAD1-add back to BPH-1 cell monolayers was quantified and compared with the attachment of MA parasites. Data shown are means of triplicate biological replicates, which were performed in triplicate ± SEM. Statistical significance was determined using a one-way ANOVA with Tukey’s multiple-comparison test.