Figure 1.
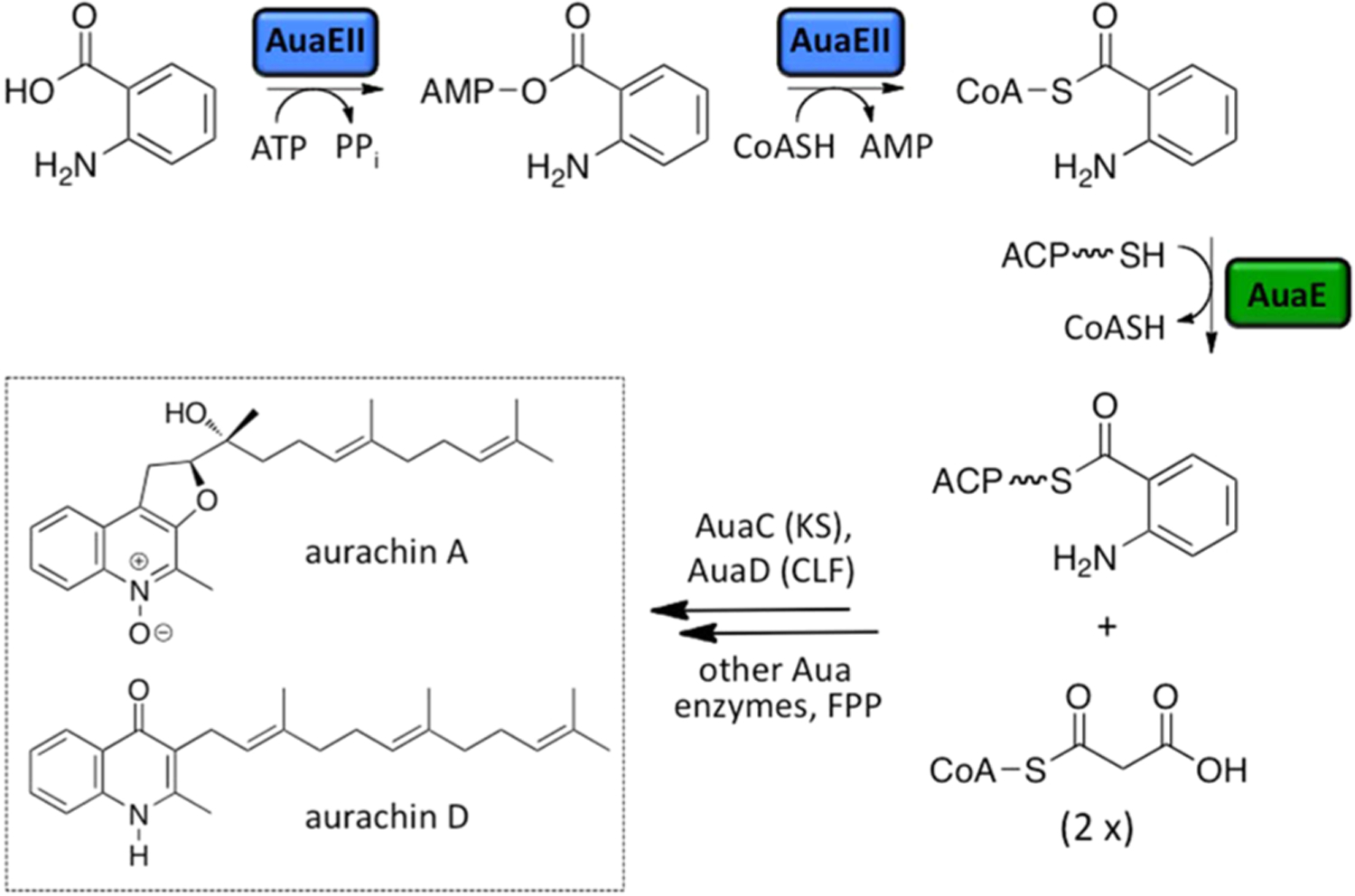
Roles of AuaEII and AuaE in aurachin biosynthesis. Aurachin biosynthesis begins with the activation of anthranilic acid into a CoA ester that is catalyzed by AuaEII. AuaEII first activates anthranilic acid using ATP to form anthraniloyl-AMP, which is susceptible to nucleophilic attack by CoA to yield anthraniloyl-CoA. AuaE uses anthraniloyl-CoA as a substrate and transfers the anthraniloyl group onto ACP (AuaB) that is then elongated by a type II PKS (AuaB, AuaC, and AuaD). Subsequent elongation and cyclization yields a quinoline core, which is modified by farnesyl group. Additional oxidation and rearrangement reactions yield a variety of aurachin natural products (only aurachin A and aurachin D are pictured).
