Abstract
In accordance with the recently released Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, there is a significant need for focused efforts on improving hemodialysis cannulation outcomes. Toward this, structured and meaningful training of our clinical personnel who cannulate in dialysis clinics is a priority. With the availability of advanced sensors and computing methods, simulators could be indispensable tools for standardized skills assessment and training. In this article we present ways in which sensor data could be used to quantify cannulation skill. As with many other medical specialties, implementation of simulator-based training holds the promise of much-needed improvement in end-stage kidney disease patient outcomes.
Keywords: cannulation, education in ESKD, ESRD, dialysis, hemodialysis, needling, quality of life, vascular access
THE CASE FOR IMPROVED CANNULATION OUTCOMES
Cannulation in patient-centered end-stage kidney disease (ESKD) care
ESKD is a medical condition that is characterized by a loss of kidney function that requires a kidney transplant or regular, long-term dialysis to sustain a patient’s life. ESKD is a large public health problem with high associated costs and morbidity and a mortality rate as high as 22% [1, 2]. In the USA, the Medicare costs associated with ESKD are enormous, accounting for ~7% of the total Medicare budget [3]. The majority of patients with ESKD initiate renal replacement therapy (RRT) via hemodialysis with peritoneal dialysis being an alternative RRT modality [4]. In Europe and the USA, hemodialysis remains a popular mode for RRT [5]. In order to perform dialysis, a vascular access must be created to the bloodstream, ideally done through the creation of an arteriovenous fistula (AVF), which is when an artery and a vein are surgically connected. Prosthetic arteriovenous grafts (AVGs) may also be used in patients who lack suitable vessels or when AVF creation fails [6]. In addition to these accesses, a tunneled dialysis catheter (TDC) may also be used to temporarily access the patient’s bloodstream for dialysis. Cannulation is the process of inserting a tube into the body for the removal or delivery of fluids. For dialysis, AVFs or grafts are cannulated in order to access the vascular system and hemodialysis patient outcomes are dependent on establishing reliable vascular access [7].
From a clinical outcomes standpoint, AVFs are the most preferred type of access due to better patient outcomes, while the TDC is the least preferred due to increased risk of infections and complications [8, 9]. Because of the benefits of AVFs, an initiative called the Fistula First Breakthrough Initiative was launched in 2004, with the goal of having a 66% rate of fistula usage nationwide in the USA [10, 11]. Consequently the rate of fistula prevalence has risen in the USA, while AVFs continue to remain the primary vascular access for hemodialysis in many European countries [12, 13]. However, the most recent Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines reflect the growing consensus that a broad ‘fistula first’ prescription may not be the most beneficial for individual patient well-being [14]. In contrast, the new KDOQI guidelines propose a patient-centered approach for ESKD care. In this paradigm, an appropriate access suitable for the patient’s unique circumstances will be identified and implemented, while also planning for future options (e.g. transplant, AVF creation) [14]. For instance, short-term TDC usage to initiate dialysis followed by preparation for dialysis with AVF, followed by potential transplant may be included in a patient’s plan.
However, one of the critical aspects that has not received adequate attention is the quality of cannulation in the clinical process of care [15]. Since increasing evidence supports that the quality of cannulation is linked to vascular access outcomes (e.g. patency), it is imperative that this factor also be subjected to similar scrutiny for innovation [15, 16]. That is, patient- and access-specific cannulation ‘pathways’ ought to be included in the clinical process of care. Based on the recent KDOQI guidelines [14], we propose that ensuring high-quality cannulation should include the following considerations:
Cannulation ought to be customized to the patient’s access: Cannulation practice should be customized to each access type and not use a one-size-fits-all approach. For instance, AVFs require different cannulation techniques than AVGs due to their different geometric and material properties. The cannulation technique will also need to be a function of AVF maturation and the access’s physical characteristics (tortuosity, diameter, etc.) [17]. The conventional method of specifying a range of angles suitable for cannulating AVFs and AVGs may not be sufficient to communicate the nuances involved in skilled cannulation of accesses [18]. Furthermore, cannulation of endo-AVFs and biological grafts may also involve adaptations to standard cannulation guidelines.
Cannulation ought to involve the most appropriate cannulator during the maturation stage of the access: Not all dialysis center staff have the requisite expertise to cannulate ‘tough’ fistulas, especially new, less-mature AVFs. As such, new fistulas should be cannulated by experienced and/or skilled staff members. Once a cannulation routine for the developing AVF is established, nonexperts may cannulate for dialysis.
Cannulation ought to involve the right technique: Traditionally, the rope-ladder, buttonhole and area techniques were used to determine where along the access the cannulator inserted the needle. As such, each of these techniques involve different needle site selection strategies and needle insertion dynamics for skilled cannulation. Recent guidelines recommend using the rope-ladder technique, while the buttonhole technique may be used judiciously when appropriate [14, 19]. Using the area technique is discouraged because of the high risk of associated complications [14].
The implementation of such cannulation pathways is essential to achieve the vision of improved patient-centered care and outcomes. Towards this, successful cannulation is defined as inserting a cannula for hemodialysis in one attempt with no infiltration and with minimal pain [14]. Less than this leads to patient discomfort, stress, trauma and, in adverse cases, a ‘cascade’ of complications.
The impact of poor cannulation
In our estimation, the following are some of the major effects of poor cannulation outcomes. First, poor cannulations have contributed to the continued use of TDCs rather than AVFs or AVGs. First cannulation in the USA of AVFs is late (∼98 days) when compared with other developed countries (∼30 days) [8, 20]. This puts the median time for first cannulation in the USA at almost twice as long as other developed countries. One factor that accounts for this difference may be the acceptance of lower blood flows; however, improvement in cannulation skills may allow for faster first cannulation of AVFs [21]. Additionally, studies have shown that an average of 4 weeks is the time needed for first cannulation of an AVF [22, 23]. In the USA, usable patent AVFs are often not cannulated until later, resulting in increased reliance on TDCs. Because of this, ~80% of patients in the USA begin dialysis with catheters, which is associated with increased mortality and morbidity [24]. Similarly, in an international study that included several European countries, 58–73% of new ESKD patients used TDCs for initiating dialysis [1]. This is similar to a study from five countries reporting that increasing the quality of cannulation—including individual skill—may result in earlier use of AVFs and decreased reliance on TDCs.
Second, poor cannulation results in access infiltration. Infiltration is the perforation of the needle through the vessel wall of the vascular access (AVF or AVG). Major infiltration can trigger a ‘cascade’ of complications that could cause loss of a usable access [25]. Even minor infiltration has adverse effects on patient health. Since needle perforation often occurs through the posterior vessel wall, it is also called ‘backwalling’ [26]. During dialysis, it is reported that minor infiltrations occur in >50% of cannulation attempts [14, 25]. About 5–7% of cannulation attempts lead to major complications because of infiltration, including hematoma formation and the potential loss of a functional vascular access. As such, needle infiltration during cannulation should be avoided to preserve the vascular access. Recent research has also pointed to the adverse effect of needle infiltration during the fistula maturation stage. Even one infiltration injury at this stage before successful two-needle cannulation is related to ~56% lower odds of maturation [15]. Another study has highlighted that intraluminal needle tip position (the position of the needle tip relative to the cross section of the AV access) is close to the center of the lumen only 9% of the time after cannulation [26]. Overwhelmingly, intraluminal needle tip positions were undesirable, since proximity of the tip to the vessel wall causes hemodynamic trauma-related complications. Therefore, infiltration-related complications are a major factor in poor cannulation outcomes.
Third, poor cannulation causes pain and trauma to patients. Patients frequently refuse AVF creation due to the fear of cannulation-related complications and pain. It has been reported that 30% of those eligible for AVFs refuse this type of access [27]. In addition to pain and bleeding associated with cannulation, patients also describe cannulators’ lack of skill as a reason for their negative opinion of AVFs [27]. Finally, in the new payment system recently announced in the USA, there are monetary incentives for safe cannulation and early removal of TDCs [28].
THE NEED FOR STRUCTURED, OBJECTIVE METRICS-BASED CANNULATION SKILLS TRAINING
Although many innovative technologies are being developed in the dialysis realm, the most important aspect of successful cannulation is the person holding the needle. Thus there is a need to develop technologies and methods for developing the skills of our cannulators.
Patients who visit dialysis centers for their weekly treatments are most likely to be cannulated by patient care technicians (PCTs), who are supervised by nurses, or by nurses [29]. Several articles have raised concern over the lack of structured, standardized and adequate training for PCTs to successfully care for patients [23, 29, 30]. For instance, instruction received by PCTs is usually didactic followed by some hands-on instruction on the use of the dialysis machine. In some cases, fake arms may be used to illustrate the ‘technical’ skills for successfully cannulating an access. However, since there is no standardized curriculum that includes hands-on training of cannulation skills, the quality of training varies among centers. A more complete training program will not only include both didactic and hands-on skills, but will also ideally be standardized to ensure a nationwide competency level among PCTs [29, 31].
The most common mode of providing hands-on skills training is the cannulation ‘fake arm’—an arm mannequin that features plastic tubes with red liquid to simulate the vascular access to be cannulated [17]. However, as a pedagogical tool, this simulator has notable limitations: the arm is unrealistic, the plastic tube does not resemble an AV access well and previous needle ‘sticks’ are readily visible on the skin. These simulators are insufficient to simulate the two most vital aspects for successful cannulation: accurate assessment of the AV access and successful needle insertion into (and from) the access. For this, a simulator needs to render key characteristics of an AV access, such as depth, tortuosity, geometry, ‘thrill’ intensity and vein fragility [17].
Some attempts have been made to develop more sophisticated simulators. The Cairns’ Simulation Experience simulator consisted of a hemodialysis machine and a high-fidelity mannequin that included other fully operational auxiliary equipment [32]. This simulator was developed to provide an immersive clinical simulation of hemodialysis and also allow for practice of emergency procedures. Several hemodialysis simulation workshops have been conducted with the Cairns’ Simulation Experience, and the evaluation of these workshops suggests high participant satisfaction with the high-fidelity simulation. Researchers note that the simulator allowed for discussion of the learning experience in a nonclinical environment, including real and challenging scenarios. Another example is the ultrasound-guided simulator for cannulation developed by Davidson et al. [33] The simulator consists of a turkey leg with a rubber tube placed on the interior in order to simulate a deep vein. Since this model uses ultrasound to guide cannulation, participants need to first become familiar with the ultrasound device (settings, image acquisition and recognition of arterial and venous vessels and anatomic structures). Users learn to cannulate via ultrasound guidance as well as fluid flashback inside the cannula [33, 34].
Thus far, however, simulators have not been developed with the goal of supporting the standardization of hands-on cannulation skills training of clinical personnel. Such a simulator would have to render realistic AV accesses in several configurations and with different geometric as well as functional characteristics. In the following section we propose that current sensor and computing technology can be utilized to create a state-of-the-art educational tool that would enable meaningful skills assessment and training.
THE POTENTIAL OF NOVEL SIMULATOR-BASED TECHNOLOGY FOR SKILLS TRAINING
In the past several years, simulation training has become widely used throughout medical education. Simulation training has been demonstrated to shorten learning time and provide a safe environment for learning [35]. For surgical training, several factors have made sensor-equipped simulators an attractive training tool, including a shift from open surgery to minimally invasive surgical procedures. Studies demonstrate that surgery residents who trained with laparoscopic ‘box’ trainers made fewer errors while performing laparoscopic cholecystectomy compared with residents who did not participate in simulation training [36–38]. Simulation-based training, if appropriately applied to dialysis vascular access, also has the potential to be effective in improving cannulation skills by providing practice for the core skills required for successful cannulation. Objective metrics obtained from sensors in the simulator (e.g. motion, force) could be used for structured assessment and training of cannulation skills.
One of the first steps in creating an effective simulator for hemodialysis cannulation is to define the core skills for safe and successful cannulation. After consultation with several expert nurse-educators, we concluded that the following three skills were the most salient for successful cannulation: palpation (for ascertaining where to insert the needle), needle entry (with the goal of obtaining flashback) and acquiring stable blood ‘flashback’ (including lowering the angle of the needle for taping). In what follows, we illustrate the potential of a novel simulator to present these key facets of cannulation skill objectively (i.e. using quantitative data) by incorporating sensors [39].
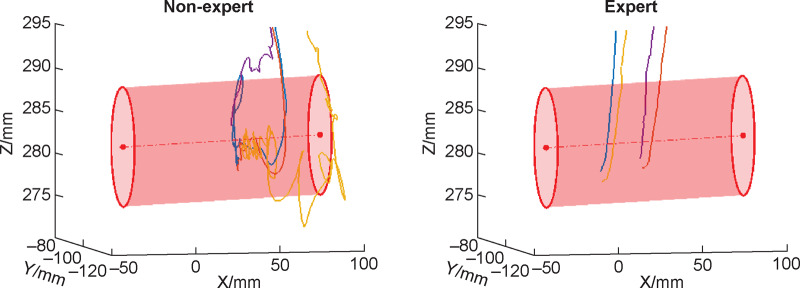
Palpation involves using one’s hands to feel the fistula as well as assess the patency of the fistula by assessing the quality of blood flow–induced ‘thrill’. The thrill is felt over the anastomosis where the vein and artery have been joined and is caused by the turbulence of the blood flow created by the arterial system merging with the venous system [18, 40]. If the thrill is felt at any location besides the anastomosis, it may be an indication of stenosis. If no thrill is present, then further evaluation is necessary. Estimation of fistula depth and curvature is also done via palpation. In short, palpation is a key step not only for assessing the health of the fistula, but also for determining where along the fistula and how to insert the needle at an appropriate angle and depth for cannulation. Figure 1 illustrates the difference in palpation path and force patterns between an expert and novice cannulator that could be used to develop benchmarks for skills training.
FIGURE 1.
Palpation behavior of a nonexpert and an expert prior to cannulating a fistula. The simulator includes four fistulas of varying characteristics, only one of which has a live ‘thrill’ at any given time. The red points indicate where the cannulator touched the skin surface, while the intensity of color corresponds to magnitude of force exerted. The blue points indicate the path traveled above the skin surface and is indicative of the process of palpation. As can be seen, the nonexpert had a longer palpation trajectory and applied greater force on the skin. The relative efficiency of the expert is clear via these data.
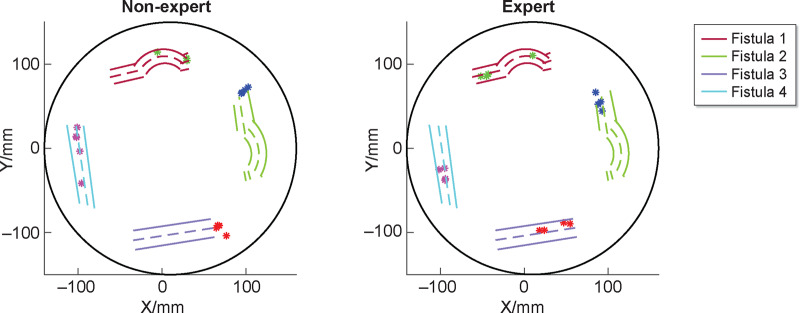
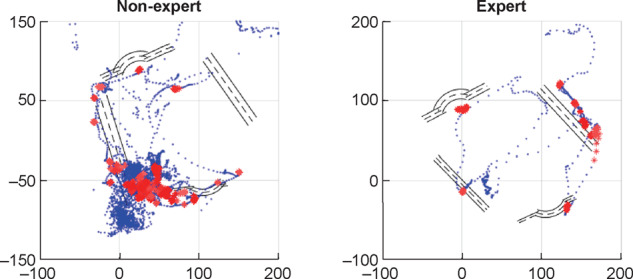
Before beginning needle insertion, a cannulator must decide on an appropriate cannulation technique. Current recommended techniques are the rope-ladder and buttonhole (to be used selectively) methods; the area technique is not to be used because of the high risk of adverse effects. For either of these methods, the location of the needle insertion is critical. Once the location is determined, the cannulator aims to obtain blood flashback by entering the fistula at an appropriate angle and depth. Needle orientation and trajectory are a function of the fistula geometry, therefore a skilled cannulator will adapt these quantitative features of cannulation according to the characteristics of the fistula. Figure 2 demonstrates the locations of needle insertion, while Figure 3 plots the trajectories of the needle with respect to the location of the fistula. This ‘objective’ data could potentially be used for skills assessment and training.
FIGURE 2.
The needle insertion trajectories of a nonexpert and expert inside the simulated fistula. The nonexpert’s needle motion is not only unsmooth, but also prone to infiltration. The expert’s motion of needle insertion is characterized by economy and efficiency.
FIGURE 3.
The locations where needles are inserted are critical during cannulation. The nonexpert’s needle site choices seem inconsistent and not ideal for optimal vascular access. In contrast, the expert’s site choices are consistent and contribute to safe cannulation.
Finally, and perhaps most importantly, the primary indicator of a successful cannulation attempt is whether or not stable flashback is obtained with no infiltration. As we have noted, infiltration needs to be avoided since even minor instances can lead to complications downstream. As such, we are currently working on a system that assesses the severity of infiltration during cannulation, which can be used as a metric for tracking the learning progress of cannulators.
In summary, in line with the recently released KDOQI guidelines, there is a significant need for focused efforts on improving hemodialysis cannulation outcomes. Towards this, structured and meaningful training of our clinical personnel who cannulate in dialysis clinics is a priority. With the availability of advanced sensors and computing methods, simulators could be indispensable tools for standardized skills assessment and training. In line with many other medical specialties, the implementation of such training holds the promise of much-needed improvement in ESKD patient outcomes.
FUNDING
Research reported in this article was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K01DK111767.
CONFLICT OF INTEREST STATEMENT
Results presented in this paper have not been published previously in whole or part, except in abstract format. R.S. reports grant from NIH/NIDDK, during the conduct of the study; Founder of Radiant Ventures, LLC. A.C. has nothing to disclose. P.R-C. is the consultant/Advisory Board member for WL Gore, BD-Bard, Medtronic, Cormedix, Humacyte, Akebia, Bayer, Vifor Pharma and Reata; Founder and CSO of Inovasc LLC. D.B-M. is an employee of Transonic Systems Inc.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
REFERENCES
- 1. Ethier J, Mendelssohn DC, Elder SJ et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2008; 23: 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronenwett JL, Johnston KW. Rutherford’s Vascular Surgery. Philadelphia: Elsevier Health Sciences, 2014 [Google Scholar]
- 3. Saran R, Robinson B, Abbott KC et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020; 75: A6–A7 [DOI] [PubMed] [Google Scholar]
- 4. Kramer A, Pippias M, Noordzij M et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary. Clin Kidney J 2019; 12: 702–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidli J, Widmer MK, Basile C et al. Editor’s choice – vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 757–818 [DOI] [PubMed] [Google Scholar]
- 6. Akoh JA. Prosthetic arteriovenous grafts for hemodialysis. J Vasc Access 2009; 10: 137–147 [DOI] [PubMed] [Google Scholar]
- 7. Riella MC, Roy-Chaudhury P. Vascular access in haemodialysis: strengthening the Achilles’ heel. Nat Rev Nephrol 2013; 9: 348–357 [DOI] [PubMed] [Google Scholar]
- 8. Rayner HC, Besarab A, Brown WW et al. Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines. Am J Kidney Dis 2004; 44: 22–26 [DOI] [PubMed] [Google Scholar]
- 9. Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int 2002; 62: 620–626 [DOI] [PubMed] [Google Scholar]
- 10. Vassalotti JA, Jennings WC, Beathard GA et al. Fistula first breakthrough initiative: targeting catheter last in fistula first Semin Dial 2012; 25: 303–310 [DOI] [PubMed] [Google Scholar]
- 11. Lok CE. Fistula First initiative: advantages and pitfalls. Clin J Am Soc Nephrol 2007; 2: 1043–1053 [DOI] [PubMed] [Google Scholar]
- 12. Allon M. Fistula First: recent progress and ongoing challenges. Am J Kidney Dis 2011; 57: 3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson BM, Akizawa T, Jager KJ et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lok CE, Huber TS, Lee T et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis 2020; 75(4 Suppl 2): S1–S164 [DOI] [PubMed] [Google Scholar]
- 15. Allon M, Imrey PB, Cheung AK et al. Relationships between clinical processes and arteriovenous fistula cannulation and maturation: a multicenter prospective cohort study. Am J Kidney Dis 2018; 71: 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parisotto MT, Schoder VU, Miriunis C et al. Cannulation technique influences arteriovenous fistula and graft survival. Kidney Int 2014; 86: 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brouwer DJ. Cannulation Camp: basic needle cannulation training for dialysis staff. Dial Transplant 2011; 40: 434–439 [Google Scholar]
- 18. Ball LK. Improving arteriovenous fistula cannulation skills. Nephrol Nurs J 2005; 32: 611–617 [PubMed] [Google Scholar]
- 19. Allon M. Is it time to abandon buttonhole cannulation of arteriovenous fistulas? Kidney Med 2019; 1: 235–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rayner HC, Pisoni RL, Gillespie BW et al. Creation, cannulation and survival of arteriovenous fistulae: data from the dialysis outcomes and practice patterns study. Kidney Int 2003; 63: 323–330 [DOI] [PubMed] [Google Scholar]
- 21. Tordoir JH, Mickley V. European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. EDTNA-ERCA J 2003; 29: 131–136 [DOI] [PubMed] [Google Scholar]
- 22. Feldman HI, Joffe M, Rosas SE et al. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 2003; 42: 1000–1012 [DOI] [PubMed] [Google Scholar]
- 23. Moist LM, Lee TC, Lok CE et al. Education in vascular access. Semin Dial 2013; 26: 148–153 [DOI] [PubMed] [Google Scholar]
- 24. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 2007; 18: 2644–2648 [DOI] [PubMed] [Google Scholar]
- 25. Lee T, Barker J, Allon M. Needle infiltration of arteriovenous fistulae in hemodialysis: risk factors and consequences. Am J Kidney Dis 2006; 47: 1020–1026 [DOI] [PubMed] [Google Scholar]
- 26. Marticorena R, Kumar L, Bachynski J et al. Ultrasound evaluation of intraluminal needle position during hemodialysis: incidental findings of cannulation complications. CANNT J 2018; 28: 39–46 [Google Scholar]
- 27. Casey JR, Hanson CS, Winkelmayer WC et al. Patients’ perspectives on hemodialysis vascular access: a systematic review of qualitative studies. Am J Kidney Dis 2014; 64: 937–953 [DOI] [PubMed] [Google Scholar]
- 28. Mehrotra R. Advancing American kidney health: an introduction. Clin J Am Soc Nephrol 2019; 14: 1788–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vachharajani TJ. Hemodialysis vascular access care in the United States: closing gaps in the education of patient care technicians. Semin Dial 2011; 24: 92–96 [DOI] [PubMed] [Google Scholar]
- 30. Dinwiddie LC, Ball L, Brouwer D et al. What nephrologists need to know about vascular access cannulation. Semin Dial 2013; 26: 315–322 [DOI] [PubMed] [Google Scholar]
- 31. Vachharajani TJ. How is arteriovenous fistula longevity best prolonged? Semin Dial 2015; 28: 24–27 [DOI] [PubMed] [Google Scholar]
- 32. Dunbar-Reid K, Sinclair PM, Hudson D. Advancing renal education: hybrid simulation, using simulated patients to enhance realism in haemodialysis education. J Renal Care 2015; 41: 134–139 [DOI] [PubMed] [Google Scholar]
- 33. Davidson IJA, Lok C, Dolmatch B et al. Virtual reality: emerging role of simulation training in vascular access. Semin Nephrol 2012; 32: 572–581 [DOI] [PubMed] [Google Scholar]
- 34. Davidson IJA, Yoo MC, Biasucci DG et al. Simulation training for vascular access interventions. J Vasc Access 2010; 11: 181–190 [DOI] [PubMed] [Google Scholar]
- 35. Widmer MK, Davidson I, Widmer LW et al. Simulation in vascular access surgery. Contrib Nephrol 2015; 184: 87–96 [DOI] [PubMed] [Google Scholar]
- 36. Fried GM. FLS assessment of competency using simulated laparoscopic tasks. J Gastrointest Surg 2008; 12: 210–212 [DOI] [PubMed] [Google Scholar]
- 37. Fried GM, Feldman LS, Vassiliou MC et al. Proving the value of simulation in laparoscopic surgery. Ann Surg 2004; 240: 518–525, discussion 525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reznick RK, MacRae H. Teaching surgical skills—changes in the wind. N Engl J Med 2006; 355: 2664–2669 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Z, Liu Z, Singapogu R. Extracting subtask-specific metrics towards objective assessment of needle insertion skill for hemodialysis cannulation. J Med Robot Res 2019; 4: 1942006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilmore J. KDOQI clinical practice guidelines and clinical practice recommendations – 2006 updates. Nephrol Nurs J 2006; 33: 487–488 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.