Figure 2.
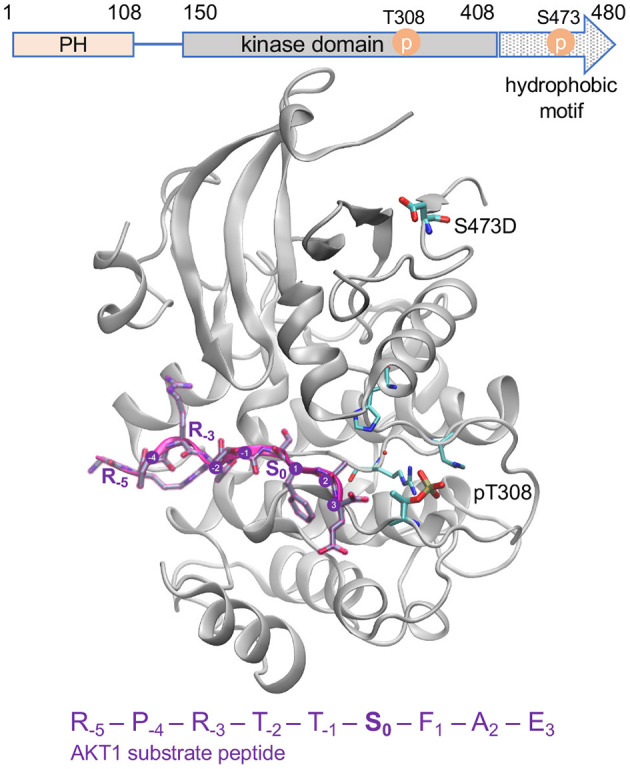
Active AKT1 bound to substrate peptide. The AKT1 structure [PDB code 3CQU (Lippa et al., 2008)] shows the kinase domain and hydrophobic motif of AKT1 (gray cartoon, residues 144–480) bound to a substrate peptide (purple). The domain organization of AKT1 is shown above. The GSK-3β derived peptide sequence is indicated, and positions surround the phosphorylation site (S0) are annotated. The phospho-accepting site (S0) is oriented in toward the active site. The structure contains pT308 and a mutation at the S473 site to aspartic acid (D). The key activating phosphorylation site, T308, is intricately connected to the active site and peptide binding site of the enzyme while the S473 site, located in the C-terminal hydrophobic motif, is distant from the active site.
