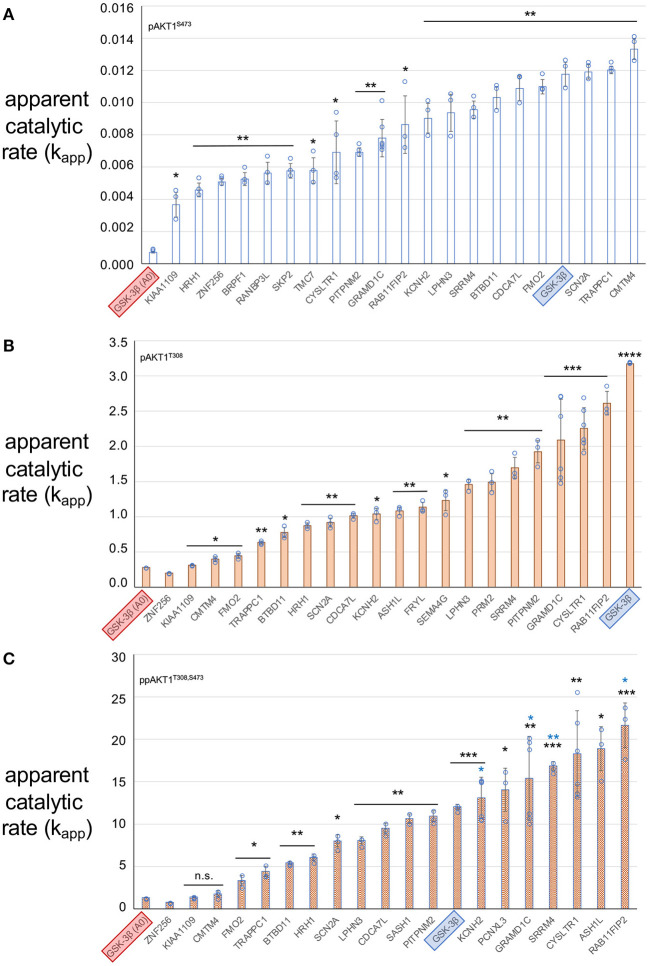
Figure 5.
Apparent catalytic rate (kapp – pmol of phospho-peptide/min/pmol of enzyme) of each AKT1 phospho-forms with peptide substrates. (A) pAKT1S473, (B) pAKT1T308, and (C) ppAKT1T308,S473. Each bar represents the average kapp value of 3 replicates and the value of each replicate is indicated by a closed circle. All three AKT1 phospho-forms were tested using the known AKT1 substrate GSK-3β (SGRPRTTSFAESCKP, blue highlight) as a standard to assess the activity of the AKT1 preparations, as well as a negative control variant of GSK-3β: GSK-3β A0 (SGRPRTTAFAESCKP, red highlight). GSK-3β A0 contains an un-phosphorylatable alanine residue in place of the serine residue that is normally phosphorylated by AKT1. Error bars show two standard deviations about mean for at least three independent reactions (N = 3 or N = 6 independent enzyme reactions). The level of statistical significance compared to GSK-3β A0 are indicated with black asterisks (*p < 0.05; **p < 0.005; ***p < 0.0005; ****p < 0.00005; n.s., not significant). Blue asterisks indicate significantly greater activity than the positive control GSK-3β peptide (*p < 0.05; **p < 0.005).