Figure 2.
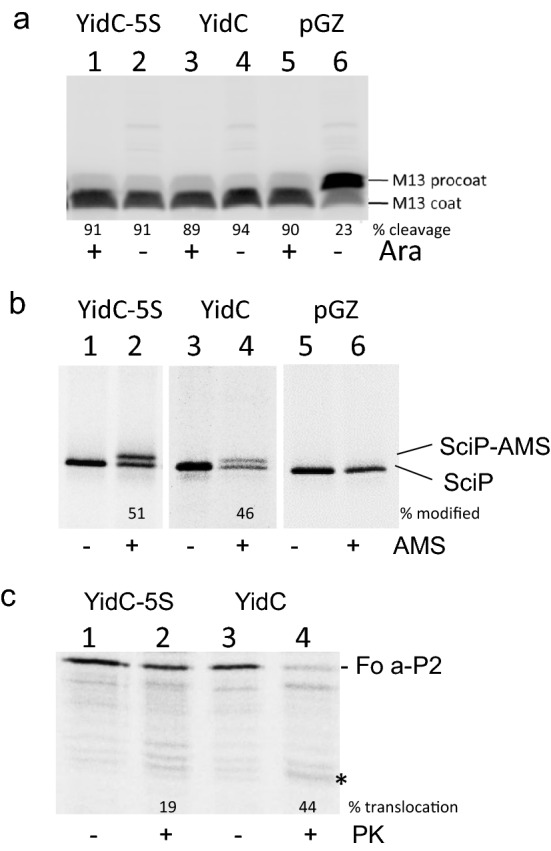
YidC-5S is fully functional for the membrane insertion of only Sec-independent substrates. The plasmid encoded YidC-5S mutant was tested for its function as a membrane insertase in E. coli MK6 under YidC-depleted conditions. A second plasmid encoding the substrate protein was co-transformed. The cells bearing the respective plasmids were grown in the presence of glucose to deplete the chromosomal YidC. As control, a culture bearing the vector (pGZ) was analysed in parallel (lanes 5, 6). (a) The expression of M13 procoat was induced with IPTG for 10 min and 35S-methionine was added for 1 min and the cells were analysed for cleavage of the M13 procoat protein to the mature coat in cells co-expressing YidC-5S (lane 2), YidC (lane 4) or with an empty plasmid (lane 6). For a control, the cleavage was followed under non-depleted conditions (+Ara, odd lanes). (b) SciP with a cysteine residue at 218 in the C-tail was co-expressed with YidC-5S (lanes 1, 2), YidC (lanes 3, 4) or where the empty plasmid was present (lanes 5, 6) and pulse-labelled for 2 min. The cells were non-treated (lanes 1, 3, 5) or treated with AMS to modify the cysteine and shift the protein by 0.5 kDa when the C-terminal tail was translocated to the periplasm (lane 2, 4, 6). (c) Membrane insertion of ATP synthase subunit a that was extended with the P2 domain of leader peptidase at the C-terminus was monitored in the YidC-depleted JS7131 cells. The cells expressing YidC-5S (lanes 1, 2) or YidC (lanes 3, 4) with subunit a-P2 were pulse-labelled with 35S-methionine for 1 min, converted to spheroplasts and analysed by protease mapping. Proteinase K was added (lanes 2, 4) or not (lanes 1, 3). When the protein was digested with Proteinase K, a protease-resistant fragment was generated (see asterisk). For a control, the cellular protection of cytosolic proOmpA and the digestion of OmpA by the protease were documented (Fig. S2). In all cases, the total cell protein was immunoprecipitated and analyzed by SDS-PAGE and phosphorimaging. The percentage of the cleavage of M13 procoat, of the modification of SciP with AMS and the protease accessibility of Fo-a-P2 were quantified and the values are indicated.
