Abstract
Background -
The genomic structure that contributes to the risk of coronary artery disease (CAD) can be evaluated as a risk score of multiple variants. However, sex differences have not been fully examined in applications of genetic risk score of CAD.
Methods -
Using data from the UK Biobank, we constructed a CAD genetic risk score based on all known loci, three mediating trait-based (blood pressure, lipids, body mass index) sub-scores, and a genome-wide polygenic risk score based on 1.1 million variants. The differences in genetic associations with prevalent and incident CAD between men and women were investigated among 317,509 unrelated individuals of European ancestry. We also assessed interactions with sex for 161 individual loci included in the comprehensive genetic risk score.
Results -
For both prevalent and incident CAD, the associations of comprehensive and genome-wide genetic risk scores were stronger among men than women. Using a score of 161 loci, we observed a 2.4 times higher risk for incident CAD comparing men with high genetic risk to men with low genetic risk, but an 80 percent greater risk comparing women with high genetic risk to women with low genetic risk. (interaction p=0.002). Of the three sub-scores, the blood pressure-associated sub-score exhibited sex differences (interaction p=0.0004 per SD increase in sub-score). Analysis of individual variants identified a novel gene-sex interaction at locus 21q22.11.
Conclusions -
Sexual differences in genetic predisposition should be considered in future studies of coronary artery disease, and genetic risk scores should not be assumed to perform equally well in men and women.
Keywords: gender differences, genetic epidemiology, coronary artery disease, risk score, the UK Biobank, interaction
Journal Subject Terms: Genetics, Cardiovascular Disease, Epidemiology
Introduction
Coronary artery disease (CAD) remains the leading cause of death in both men and women in the US.1 The incidence, clinical presentation and mortality of CAD differ markedly between men and women. For example, young and middle-aged women are relatively protected from developing an acute myocardial infarction (MI) compared with men and develop the disease at an older age, women with early-onset MI have a poorer prognosis relative to men.2, 3 Despite growing observations of sex difference in CAD, the disparity cannot be fully explained by differences in conventional risk factors.4 Better understanding of the molecular mechanisms underlying the sex disparity of cardiac and vascular pathophysiology in CAD may improve the prevention and management strategies of CAD risk in men and women.
CAD is a heritable condition and has a strong genetic component.5 Recent genome-wide association studies (GWAS) have identified over 100 CAD-associated genetic loci across the genome and demonstrated a polygenic architecture of CAD.5, 6 However, most loci have small effect size and fail to appreciably account for a large proportion of CAD heritability, which motivated the discovery of other genetic components such as gene-environment interactions.7 Sex may represent important differences in external and internal environment between men and women, however, to which extent the genetic predisposition to CAD differ between men and women remains unclear. Previous evidence has shed light on gene-sex interaction for CAD8 but the results have been inconsistent.9–11 Our study is aimed at exploring whether the genetic effect on CAD development is modified by sex in a large population of European ancestry.
Methods
The data that support the findings of this study is available through appropriate application to the UK Biobank. Methods used in the analysis including computational codes are available from the corresponding author upon reasonable request.
The UK Biobank study received ethics approval from the NHS National Research Ethics Service North West (reference number: 16/NW/0274). Data access permission for this study was granted under UKB application 34031.
Full methods are now available in Supplementary Materials.
Results
Characteristics of the study population are summarized in Table 1. Prevalence of smoking, alcohol consumption, hypertension, diabetes, and use of lipid medications were higher among men compared with women. We identified 10,358 prevalent CAD cases (77.0% men) at enrollment. After enrollment, 9,847 primary incident CAD events occurred (68.8% men) during a median follow-up time of 6.1 (range 0 – 8.4) years.
Table 1.
Basic characteristics of 317,509 UK Biobank participants of European ancestry
| Characteristic | Mean (SD) or N (%) | ||
|---|---|---|---|
| All N=317,509 | Males N=146,246 (46.1%) | Females N=171,263 (53.9%) | |
| Prevalent CAD | 10,358 (3.3%) | 7,976 (5.5%) | 2,382 (1.4%) |
| Incident CAD* | 9,847 (3.2%) | 6,774 (4.9%) | 3,073 (1.8%) |
| Age | 57.4 (8.0) | 57.6 (8.1) | 57.2 (7.9) |
| BMI | 27.4 (4.7) | 27.8 (4.2) | 27.0 (5.1) |
| Smoking Status | |||
| Current | 30,468 (9.6%) | 16,673 (11.4%) | 13,795 (8.1%) |
| Past | 112,607 (35.5%) | 57,709 (39.5%) | 54,898 (32.1%) |
| Never | 174,434 (54.9%) | 71,864 (49.1%) | 102,570 (59.9%) |
| Alcohol Consumption | |||
| Ever | 307,932 (97.0%) | 143,889 (98.4%) | 164,043 (95.8%) |
| Never | 9,577 (3.0%) | 2,357 (1.6%) | 7,220 (4.2%) |
| Hypertension | 85,340 (26.9%) | 44,459 (30.4%) | 40,881 (23.9%) |
| Diabetes | 14,923 (4.7%) | 9,207 (6.3%) | 5,716 (3.3%) |
| Lipid Lowering Medications | 54,616 (17.2%) | 33,423 (22.9%) | 21,193 (12.4%) |
| Education | |||
| School leaving age >=15 | 249,292 (78.5%) | 114,256 (78.1%) | 135,036 (78.8%) |
| School leaving age <15 | 68,217 (21.5%) | 31,990 (21.9%) | 36,227 (21.2%) |
| Townsend Index | −1.6 (2.9) | −1.6 (3.0) | −1.6 (2.9) |
CAD incidence was calculated excluding prevalent CAD cases at enrollment (N=307,151).
Both CAD-GRS based on 161 known loci and genome-wide CAD-LDpred were associated with prevalent CAD at baseline (Table 2) as well as incident primary CAD after enrollment (Table 3), and the associations were stronger in men than women. For primary incident CAD, men with high CAD-GRS had over two-fold higher risk comparing to men with low CAD-GRS (HR: 2.39, 95% CI: 2.21 – 2.59), while women with high CAD-GRS had eighty percent higher in risk comparing to women with low CAD-GRS. (HR: 1.82, 95% CI: 1.62 – 2.05, interaction p=0.002). (Figure 1A) Using CAD-LDpred, the genetic association with incident CAD was stronger among men (HR: 2.55, 95% CI: 2.35 – 2.77) than women (HR: 1.95, 95% CI: 1.73 – 2.19, interaction p=0.007) (Figure 1B). In the analysis for three mediating trait-based CAD-sub-GRSs, we only observed gene-sex interaction for GRSCAD-BP with incident CAD where men had stronger association than women but no significant interaction was observed for GRSCAD-lipids or GRSCAD-BMI (Table 3). We also tested the interaction of each CAD-sub-GRS with sex for prevalent and incident CAD in a stepwise manner. The initial model included all three CAD-sub-GRSs as well as their interactions with sex. Then in each following step, the least significant sub-GRS interaction with sex was dropped. Results from the stepwise interaction testing for three CAD-sub-GRSs (Supplement, eTable 1 and eTable 2) were consistent with sex-interaction analysis when each CAD-sub-PRS was analyzed on its own.
Table 2.
Associations of polygenic and genetic risk scores of coronary artery disease with prevalent CAD in the UK Biobank
| Genetic predisposition* | All OR (95% CI) | Men OR (95% CI) | Women OR (95% CI) | Interaction P-value | |
|---|---|---|---|---|---|
| CAD-GRS | Per SD increase | 1.41 (1.38, 1.44) | 1.44 (1.40, 1.48) | 1.33 (1.27, 1.39) | 3×10−4 |
| High | 2.54 (2.36, 2.73) | 2.66 (2.44, 2.89) | 2.23 (1.92, 2.57) | ||
| GRSCAD-BP | Per SD increase | 1.15 (1.12, 1.17) | 1.14 (1.11, 1.17) | 1.16 (1.11, 1.20) | 0.76 |
| High | 1.43 (1.33, 1.53) | 1.39 (1.29, 1.51) | 1.52 (1.33, 1.73) | ||
| GRSCAD-lipids | Per SD increase | 1.29 (1.27, 1.32) | 1.31 (1.28, 1.34) | 1.23 (1.18, 1.28) | 3×10−3 |
| High | 2.02 (1.89, 2.16) | 2.09 (1.94, 2.26) | 1.80 (1.57, 2.06) | ||
| GRSCAD-BMI | Per SD increase | 1.07 (1.05, 1.09) | 1.08 (1.05, 1.10) | 1.05 (1.01, 1.10) | 0.29 |
| High | 1.16 (1.09, 1.24) | 1.18 (1.09, 1.28) | 1.12 (0.98, 1.28) | ||
| CAD-LDpred | Per SD increase | 1.44 (1.41, 1.47) | 1.47 (1.43, 1.51) | 1.37 (1.31, 1.43) | 8×10−4 |
| High | 2.74 (2.54, 2.95) | 2.92 (2.67, 3.19) | 2.27 (1.96, 2.63) | ||
When scores were categorized, participants with low genetic risk (quintile 1) for each score were used as the reference group
Table 3.
Associations of polygenic and genetic risk scores of coronary artery disease with primary incident CAD in the UK Biobank
| Genetic predisposition* | All HR (95% CI) | Men HR (95% CI) | Women HR (95% CI) | Interaction P-value | |
|---|---|---|---|---|---|
| CAD-GRS | Per SD increase | 1.34 (1.31, 1.36) | 1.38 (1.34, 1.41) | 1.25 (1.21, 1.30) | 5×10−5 |
| High | 2.20 (2.06, 2.35) | 2.39 (2.21, 2.59) | 1.82 (1.62, 2.05) | ||
| GRSCAD-BP | Per SD increase | 1.14 (1.12, 1.16) | 1.17 (1.14, 1.20) | 1.08 (1.04, 1.12) | 4×10−4 |
| High | 1.42 (1.34, 1.51) | 1.54 (1.43, 1.66) | 1.19 (1.06, 1.33) | ||
| GRSCAD-lipids | Per SD increase | 1.18 (1.16, 1.21) | 1.19 (1.17, 1.22) | 1.16 (1.12, 1.20) | 0.17 |
| High | 1.57 (1.47, 1.67) | 1.60 (1.48, 1.73) | 1.49 (1.34, 1.67) | ||
| GRSCAD-BMI | Per SD increase | 1.08 (1.06, 1.10) | 1.07 (1.05, 1.10) | 1.08 (1.05, 1.12) | 0.47 |
| High | 1.25 (1.17, 1.33) | 1.25 (1.16, 1.35) | 1.24 (1.10, 1.38) | ||
| CAD-LDpred | Per SD increase | 1.36 (1.33, 1.39) | 1.39 (1.36, 1.43) | 1.29 (1.24, 1.33) | 3×10−3 |
| Intermediate | 1.51 (1.42, 1.60) | 1.59 (1.47, 1.71) | 1.33 (1.20, 1.49) | 7×10−3 | |
| High | 2.35 (2.20, 2.51) | 2.55 (2.35, 2.77) | 1.95 (1.73, 2.19) | ||
When scores were categorized, participants with low genetic risk (quintile 1) for each score were used as the reference group
Figure 1.
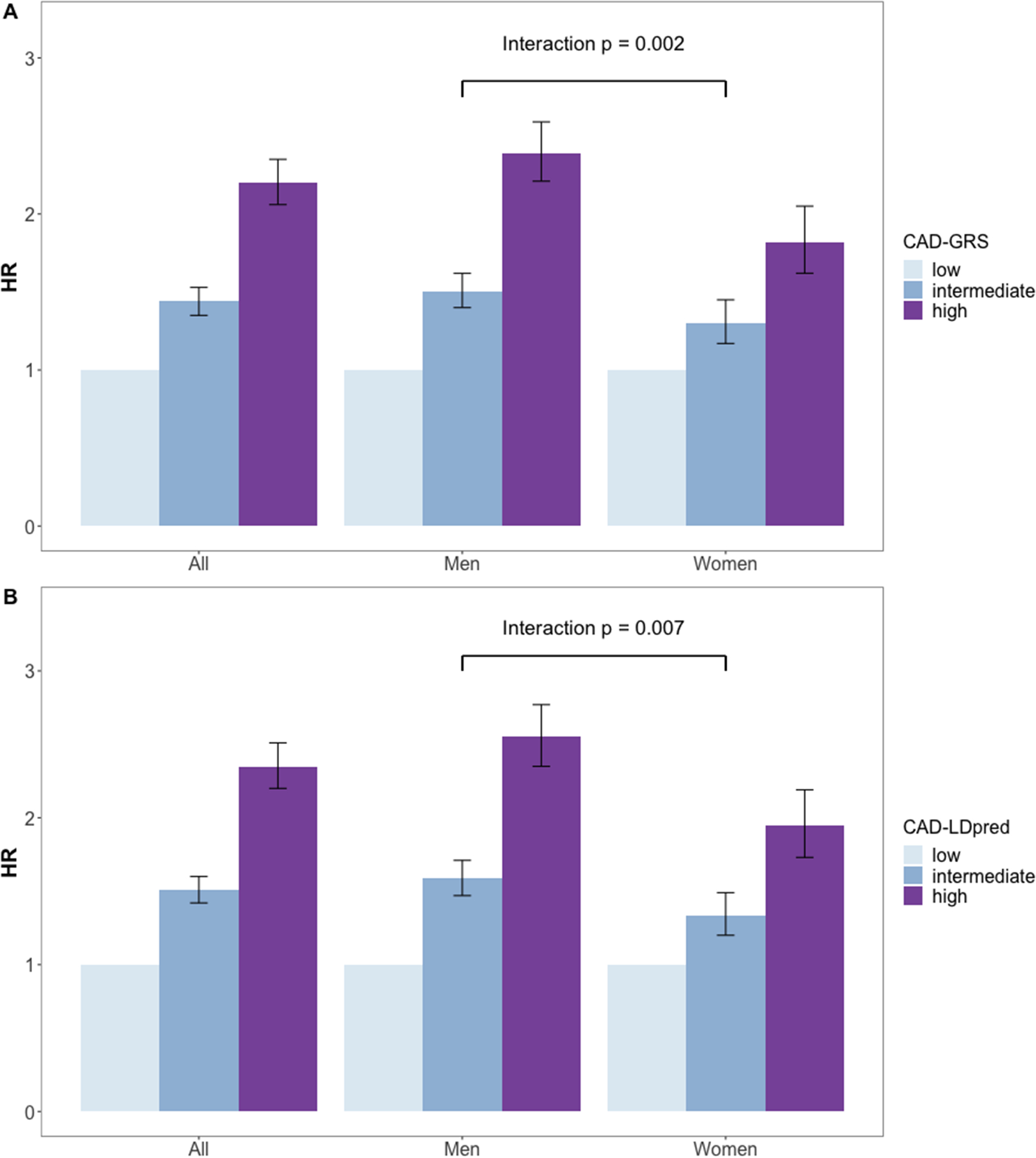
Associations of known loci-based CAD genetic risk scores (CAD-GRS) and a genome-wide polygenic score (CAD-LDpred) with incident CAD in the UK Biobank. 1-A: Association of CAD-GRS with incident CAD, 1-B: Associations of CAD-LDpred with incident CAD, HR: hazard ratio.
Women tend to develop CAD at later age than men. To test if sex-interaction pertains among older individuals, we also examined sex interaction with CAD-GRS and CAD-LDpred for incident CAD by restricting to 119,656 individuals with age at enrollment > 60 years. In total, 6,216 incident CAD cases developed. Overall, we still observed GRS-sex interaction with consistent magnitude but less significant effects due to reduced power. (Supplement, eTable 3) Among those who were older than 60 years at enrollment, men with higher genetic risk had greater increase in CAD risk (HR per SD increase in CAD-GRS: 1.33, 95% CI: 1.29, 1.37) compared to women (HR per SD increase in CAD-GRS: 1.23, 95% CI: 1.18, 1.29, interaction p=0.02).”
Among the 161 loci included in our CAD-GRS, (Supplement, eTable 4 and eTable 5) only one locus (21q22.11) had a significant SNP-sex interaction effect with incident CAD (p = 1×10−4) after adjusting for multiple testing (Bonferroni correction threshold: p = 0.05/161 = 3×10−4) (Figure 2). The lead SNP rs28451064 is a cis-eQTL for multiple genes including MRPS6, SLC5A3, KCNE2 and AP000318.2 expressed in the aorta and tibial arteries.12 We also tested sex-interaction for CAD-GRS and GRSCAD-BP excluding rs28451064 with incident CAD. The sex-interaction persisted but attenuated, indicating that the sexual heterogeneity observed for both CAD-GRS and GRSCAD-BP was partially explained by the 21q22.11 locus. (Supplement, eTable 6)
Figure 2.
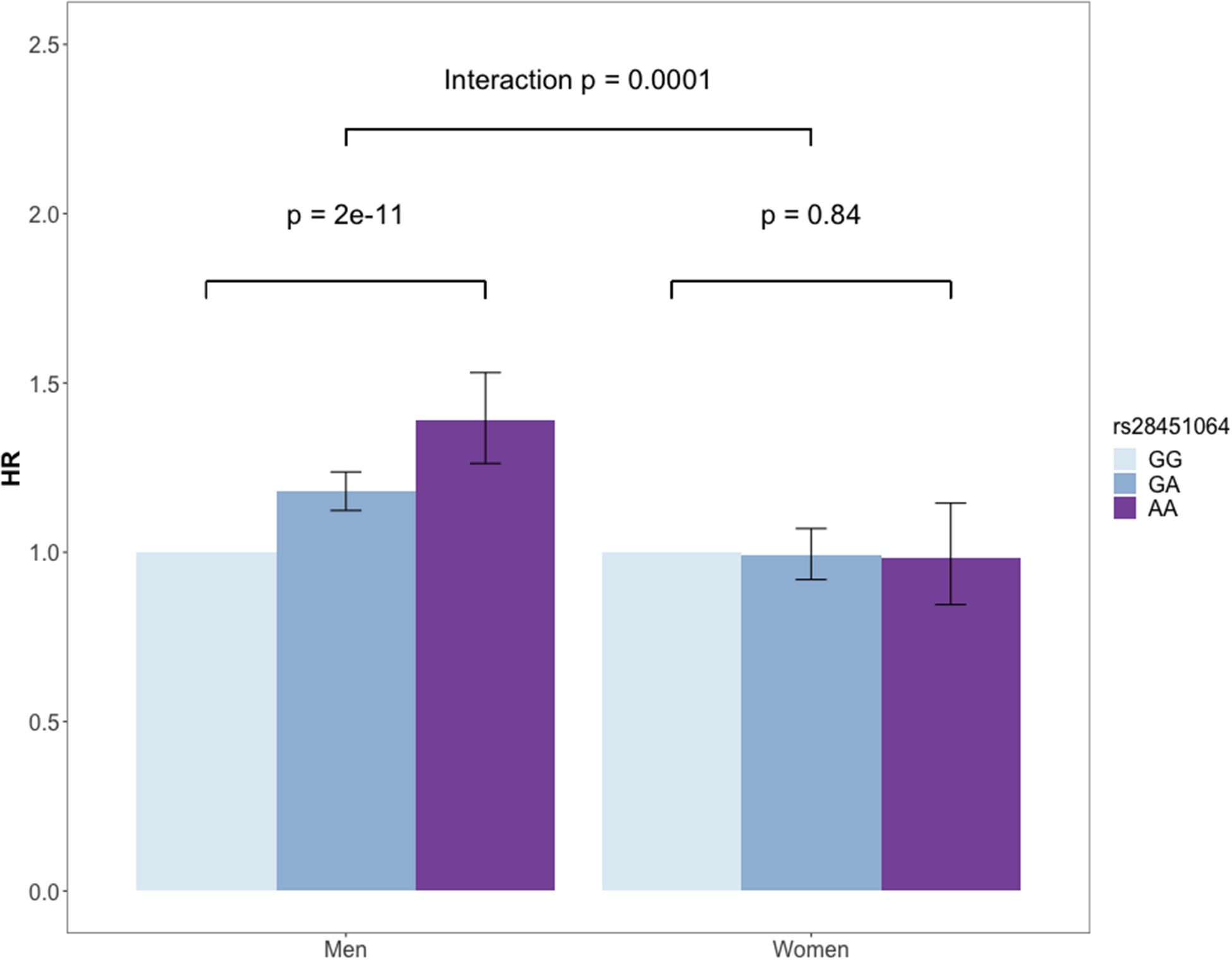
Effect of rs28451064 on incident CAD in the UK Biobank by sex. The genetic association was tested using an additive model; HR: hazard ratio, HR for men: 1.18, 95% CI: 1.12, 1.24; HR for women: 0.99, 95% CI: 0.92, 1.07.
Discussion
Genetic contributions to sex differences in CAD risk are not well understood. Using CAD-GRS, we observed higher genetic risk for CAD in men than that in women in a large European cohort from UK Biobank. We observed significant GRS-sex interaction for GRSCAD-BP, but not for GRSCAD-lipids or GRSCAD-BMI. Such differences in sub-GRS of CAD suggest that blood pressure-related but not BMI- or lipids-related genes might contribute to a key mechanism driving the sex disparity of CAD via interaction with the genetic susceptibility of CAD. We also identified one locus (21q22.11) with significant SNP-sex interaction for incident CAD and eQTL mapping implicated several genes including MRPS6, SLC5A3, KCNE2 and AP000318.2 in arterial tissues.12 This locus has also been shown to be associated with bone mineral density, waist-hip-ratio and pulse pressure, traits with known sex disparities.13–15
The UK Biobank is a very large population-based biobank cohort with clinical follow-up data obtained from hospital records. A previous study from the UK Biobank reported no GRS-sex interaction on cumulative incidence of CAD by age when using a metaGRS containing over 1 million SNPs.16 However, our study that has focused on a more strictly defined unrelated sample of European ancestry and used calendar time to measure CAD events revealed potentially missed gene-sex interaction for CAD development. In addition, our sub-score and individual loci analyses suggest that BP-related loci might contribute to the sex differences in genetic predisposition of CAD.
Sex-related risk including behavioral, socioeconomic and biological factors for developing CAD can change over time. The cross-sectional analysis of prevalent cases was subject to more bias compared to the analysis of incident cases where risk factors preceding disease onset could be controlled for, particularly for those sex-related risk factors. In addition, we compared the age and sex distribution between prevalent and incident CAD cases. We observed that prevalent cases had younger age of onset (median: 58.5 years, range: 32.7–70.1 years) compared to incident cases (median: 66.4 years, range: 41.4–77.4 years), and lower proportion of female cases (23% vs. 31%). These differences can also potentially explain the inconsistency of sex-interaction for CAD-sub-GRS we observed between prevalent vs. incident cases. For gene-environment interaction analysis in general, we recommend a prospective design to investigate risks for developing incident cases whenever possible. Large biobank studies such as UK Biobank offer a unique opportunity of prospective study of gene-environment and gene-risk factor interactions by extracting incident disease cases from longitudinal electronic health record database.
Our study also has several limitations. CAD incidence based on in-patient hospital records may be underestimated; however, this bias would not be expected to differ between men and women. Our study population consists of relatively older participants (57.4±8.0 years old) at baseline, so it is not well suited to assess the genetic contribution to early-onset CAD. However, we observed consistent sex-interaction of comprehensive CAD-GRS and CAD-LDpred between prevalent and incident CAD which suggests gene-sex interaction should also be considered for early-onset CAD. Prospective studies of younger populations that capture early-onset CAD events would help improve our understanding of gene-sex interactions in CAD risk.
Our study suggests that the genomic susceptibility for developing CAD is greater in men than in women. In addition to the findings in the GRS categories, the same genomic risk dosage (per SD increase in CAD-GRS) in men translates into a 1.1 fold higher risk for developing incident CAD events than in women. The sexual difference in genetic predisposition of CAD may partially explain the higher CAD risk among adult men than women. Our results emphasize the importance of evaluating context dependence of GRS when applied to evaluation of genomic risk for CAD. Integration of genetic interactions with CAD risk factors would further improve the precise understanding of CAD risk across the population with heterogeneous risk profiles. Applying such genetic interaction analyses in other cardiovascular disease (CVD) outcomes may also be critical to address health disparity of CVD, which is an increasing challenge of public health and medicine. Our results emphasize the importance of evaluating context dependence of GRS when applied to evaluation of genomic risk for CAD.
Supplementary Material
Sources of Funding:
This research has been conducted using the UK Biobank Resource under Application Number “34031”.
Non-standard Abbreviations and Acronyms
- CAD
coronary artery disease
- MI
myocardial infarction
- UKB
the UK Biobank
- HES
hospital episode statistics
- OPCS-4
Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4
- CAD-GRS
CAD genetic risk score of 161 loci
- CAD-sub-GRS
mediating trait-based CAD sub-genetic risk score
- GRSCAD-lipids
lipids-based CAD sub-genetic risk score
- GRSCAD-BP
blood pressure-based CAD sub-genetic risk score
- GRSCAD-BMI
BMI-based CAD sub-genetic risk score
- CAD-LDpred
genome-wide CAD polygenic risk score calculated using LDpred
Footnotes
Disclosures: None.
References:
- 1.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158:2054–62. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–25. [DOI] [PubMed] [Google Scholar]
- 4.Wingard DL, Suarez L, Barrett-Connor E. The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol. 1983;117:165–72. [DOI] [PubMed] [Google Scholar]
- 5.van der Harst P, Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2018;122:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy JJ. Gene by sex interaction in the etiology of coronary heart disease and the preceding metabolic syndrome. Nutr Metab Cardiovasc Dis. 2007;17:153–61. [DOI] [PubMed] [Google Scholar]
- 9.Silander K, Alanne M, Kristiansson K, Saarela O, Ripatti S, Auro K, Karvanen J, Kulathinal S, Niemelä M, Ellonen P, et al. Gender differences in genetic risk profiles for cardiovascular disease. PloS one. 2008;3:e3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu LY, Schaub MA, Sirota M, Butte AJ. Sex differences in disease risk from reported genome-wide association study findings. Hum Genet. 2012;131:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orozco G, Ioannidis JP, Morris A, Zeggini E. Sex-specific differences in effect size estimates at established complex trait loci. Int J Epidemiol. 2012;41:1376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguet F, Barbeira AN, Bonazzola R, Brown A, Castel SE, Jo B, Kasela S, Kim-Hellmuth S, Liang Y, Oliva M, et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. bioRxiv 787903; doi: 10.1101/787903 [DOI] [Google Scholar]
- 13.Kelly PJ, Twomey L, Sambrook PN, Eisman JA. Sex differences in peak adult bone mineral density. J Bone Miner Res. 1990;5:1169–75. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Engstrom G, Hedblad B, Calling S, Berglund G, Janzon L. Sex differences in the relationships between BMI, WHR and incidence of cardiovascular disease: a population-based cohort study. Int J Obes (Lond). 2006;30:1775–81. [DOI] [PubMed] [Google Scholar]
- 15.Rogers RG, Onge JM. Race/ethnic and sex differentials in pulse pressure among us adults. Ethn Dis. 2005;15:601–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults: Implications for Primary Prevention. J Am Coll Cardiol. 2018;72:1883–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


