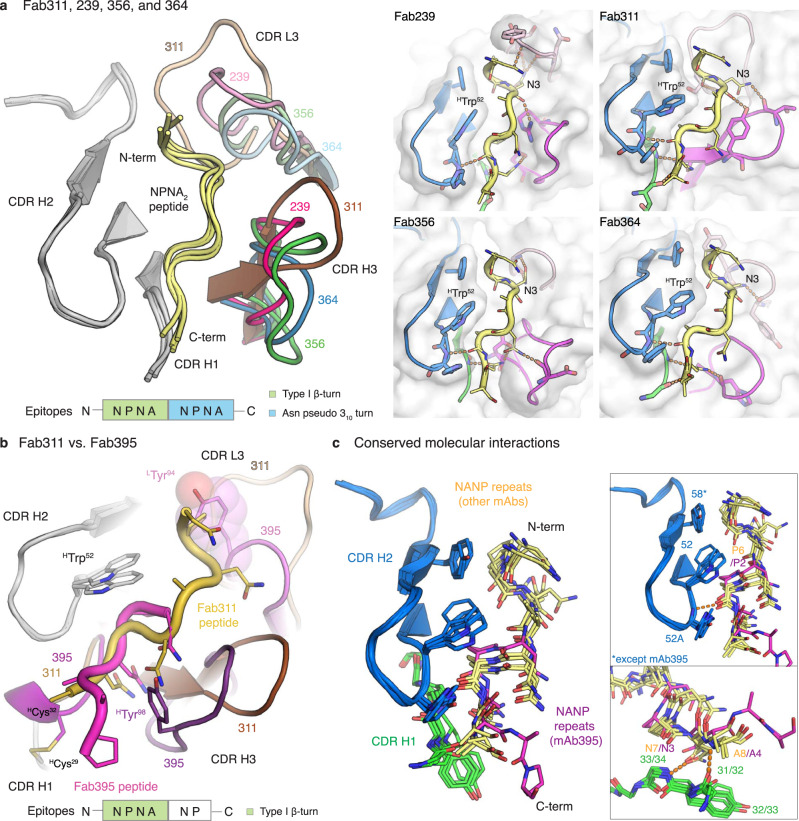
Fig. 4. Crystal structure of IGHV3-33 Fabs.
a Structures of Fab239, 311, 356, and 364 in complex with NPNA2 peptide (yellow) are shown in cartoon representation and aligned based on CDR H2. Only the CDRs involving in peptide binding are shown with CDR H1 and H2 colored in grey and CDR H3 and L3 colored for the different antibodies as indicated. A schematic of the epitope structural motifs is also indicated below. Close-up views of the paratopes are also displayed with the Fabs as cartoons embedded in their surface representation. CDR H1, H2, H3, and L3 are colored green, blue, magenta, and pink, respectively, and the peptides are shown as yellow tubes with side chains in stick representation. Antibody side chains engaging in hydrogen bonds (orange dashes) and key interacting aromatic residues are also shown as sticks. b The paratopes of Fab395 aligned to that of Fab311 based on CDR H2 (grey) are displayed as cartoons with their CDRs colored as shown, with the schematic of the Fab395 epitope structural motif also indicated below. The side chains of HTrp52, HTyr98, and LTyr94, HCys29, and HCys32 are highlighted as sticks (also with a surface representation for LTyr94). The peptides are shown as tubes with side chains as sticks and colored as indicated. c Side chains of residues involving in conserved molecular interactions from CDR H1 (green) and H2 (blue) and the peptides are shown as sticks. The peptide bound to Fab395 is colored magenta, whereas others are in yellow. Hydrogen bonds are displayed as orange dashes.