Figure 6.
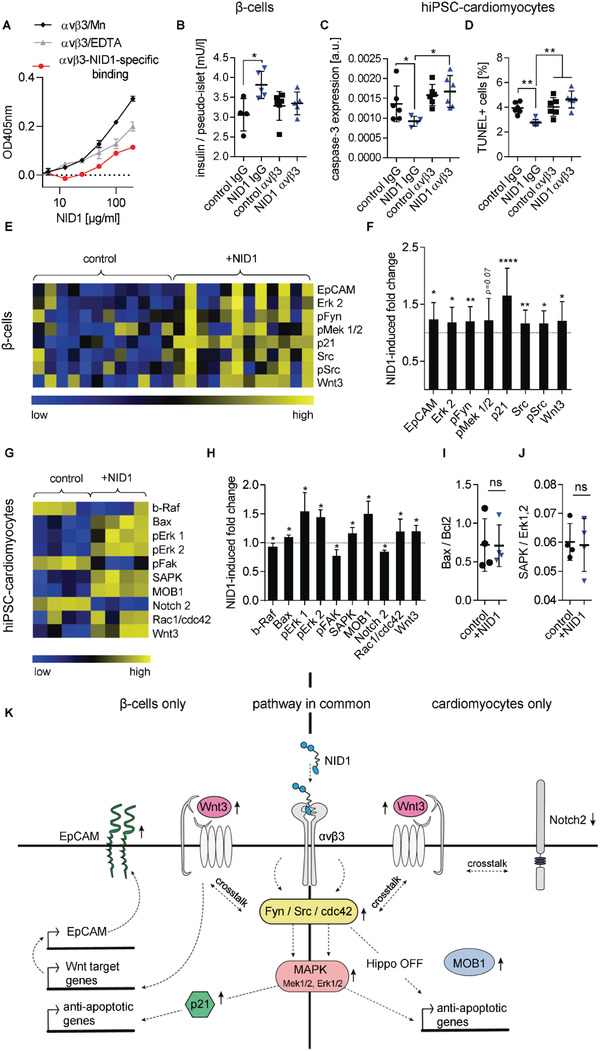
NID1 signals via αvβ3 and activates the MAPK pathway in β‐cells and cardiomyocytes. A) Divalent cation‐dependent and dose‐dependent binding of αvβ3 with NID1. B) Blocking of αvβ3 in NID1‐treated and control pseudoislets under normoxic conditions. Functionality assessment by glucose stimulation at 20 × 10−3 m glucose (n ≥ 4); one‐way ANOVA with Tukey's multiple comparisons test. c,d) Blocking of αvβ3 in NID1‐treated and control hiPSC‐CMs by a αvβ3 antibody under hypoxic conditions. Protective effect of NID1 assessed by C) cleaved caspase‐3 (n ≥ 4) and D) TUNEL assay (n = 6); one‐way ANOVA with Tukey's multiple comparisons test. E–H) DigiWest‐based protein expression analysis of significantly regulated proteins in NID1‐treated E,F) pseudoislets (n = 12) and G,H) hiPSC‐CMs (n = 4) under hypoxic conditions compared with their respective controls. Data are (E,G) shown as column‐wise and color‐coded heatmap from the lowest (blue) to the highest (yellow) expression for each analyte; and (F,H) quantified as fold change induced by NID1. Nonparametric Wilcoxon Rank sum test. I,J) Protein ratios of (I) Bax to Bcl2 and (J) SAPK to Erk1,2 (n = 4); unpaired t‐test. K) Proposed common and separate mechanisms of action of NID1 for CMs and β‐cells in vitro. Middle: common pathway, NID1‐αvβ3 ligation upregulating Fyn/Src in β‐cells and cdc42 in CMs, which stimulates Wnt3 and the MAPK pathway. Left: β‐cells, Fyn/Src activates the MAPK pathway, upregulating p21, which can be antiapoptotic. Fyn/Src crosstalks with Wnt3 and EpCAM, which enhances insulin secretion. Right: CMs, cdc42 downregulates the Hippo pathway as shown by an upregulation of MOB1. Pathways specific for each cell type are shown either on the left for β‐cells and on the right for CMs. *p < 0.05; **p < 0.01, ***p < 0.001, and ****p < 0.0001.
