Abstract
Noda epileptic rat (NER) is a mutant model for epilepsy that exhibits spontaneous generalized tonic-clonic seizure. Epileptogenesis of NER remains to be elucidated; but it is detected an insertion of an endogenous retrovirus sequence in intron 2 of the PHD finger protein 24 (Phf24) gene, encoding Gαi-interacting protein (GINIP). Phf24 is a strong candidate gene for epileptogenesis in NER. PHF24 modulates GABAB signaling through interacting with Gαi protein. To clarify the epileptogenesis of NER, we investigated a distribution of PHF24-expressing cells in the central nerve system (CNS). While broad expression of PHF24 was observed in the CNS, characteristic expression was noted in the periglomerular layer of the olfactory bulb and the lamina II of the spinal cord in the control rats. These cells showed co-expression with calbindin or calretinin, inhibitory interneuron markers. In the olfactory bulb, 15.6% and 41.2% of PHF24-positive neurons co-expressed calbindin and calretinin, respectively. Immunoelectron microscopy revealed that PHF24 was located in the presynaptic terminals, synaptic membranes and cytoplasmic matrix of neuronal soma. Our data suggested PHF24 is expressed in the inhibitory interneurons and may play important roles in modulation of the GABAB signaling.
Keywords: epilepsy, GABAB receptor, inhibitory interneuron, mutant rat, PHF24
Introduction
Noda epileptic rat (NER) is a mutant model for epilepsy discovered in the colony of Crj:Wistar in Japan and exhibits spontaneous generalized tonic-clonic seizure [1]. First convulsion occurs as early as 8 weeks and subsequently spontaneous convulsions are observed almost every 30 h. That convulsion begins with neck and forelimb clonus, wild jumping/running, opisthotonic posturing, and evolving to tonic, then clonic convulsion. Cortical and hippocampal electroencephalograms during seizure show characteristic high voltage diffuse spikes and spike-and-wave complexes, and these electroencephalograms are comparable to human epilepsy [1]. The seizure of NER originates from forebrain and is induced primarily by activation of limbic and/or cortical seizure circuits [2]. Therefore, NER may be classified as temporal lobe epilepsy model.
Previous study demonstrated the abnormal excitability in the brain of NER, which is potentially associated with the insufficient GABAergic neuron activity [3]. Kiura et al. reported that abnormal excitability of CA3 pyramidal neurons is related to dysfunction of Ca2+ channels in NER [4]. However, the detailed mechanisms of the seizure in NER remain to be elucidated.
Three candidate genes were identified to contribute to the epileptogenesis of NER [5, 6]. PHD finger protein 24 (Phf24) was identified as a major candidate gene; lack of PHF24 expression was considered to be related to epileptogenesis of NER [6]. PHF24 is a key modulator of peripherally evoked GABAB-receptor signaling [7]. It interacts physically with solely Gαi protein and plays an important role in downstream signaling related to Gαi protein in the dorsal ganglion cells. It is widely accepted that Gαi protein inhibits the production of cAMP. Low concentration of cAMP caused by GABAB signaling delays the recruitment of synaptic vesicles at presynaptic terminals [8]. Intense interference to high voltage activated Ca2+ channels and slight affection to production of cAMP were indicated as response function of PHF24 to Gαi coupled receptors. PHF24-null mouse showed impaired sensitiveness to GABAergic stimulation evoked with baclofen, a GABAB receptor agonist, in the spared nerve injury model that induces neuropathic pain [7]. PHF24-deficient dorsal ganglion neurons exhibited incomplete GABAergic inhibitory signaling to the interneurons in the lamina IIi in the spinal cord. Lui et al. showed that peripheral nerve axotomy causes loss of PHF24 in dorsal ganglion cells, and it related to allodynia and hyperalgesia that result from peripheral nerve axotomy [9].
In order to clarify the further role of PHF24 in the central nerve system (CNS), we investigated the detailed distribution of PHF24 positive cells in the CNS. Our results demonstrated that interneurons in the spinal cord and olfactory bulb densely expressed PHF24 as well as inhibitory interneuron markers calbindin and calretinin. In the NER and PHF24-null rats, PHF24 expression was markedly decreased in the spinal cord and olfactory bulb. Our data suggested PHF24 might be associated with the function of interneurons. It is likely that the loss of PHF24 induces functional disorders of interneuron, being involved in epileptogenesis of NER.
Materials and Methods
Animals
The NER/Kyo (NBRP#0010) strain used in this study was supplied by the National BioResource Project-Rat, Kyoto University (Kyoto, Japan). F344-Phf24em2kyo (PHF24-null rat; NBRP#0749) was generated by the TALENs as previously reported [10]. We examined homozygous NER at 20 weeks of age and PHF24-null rats at 15 weeks of age [10]. F344/Stm rats (F344; NBRP#0140) were also obtained from the National BioResource Project-Rat at 15 weeks of age were used as non-affected control. Three animals were examined in each group. All rats were maintained under SPF conditions in a room with controlled temperature and 12 h light-dark cycle at the Education and Research Center for Experimental Animal Science of Osaka Prefecture University. Food and water were provided ad libitum. All animals were handled according to the Guidelines for Animal Experimentation, Osaka Prefecture University.
Immunohistochemistry
Rats were deeply anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde (PFA) and 0.1% glutaraldeyde in 0.1 M phosphate buffer (PB; pH7.4). Tissue samples from cervical and thoracic spinal cords and brain were routinely processed and embedded in paraffin wax. The four-µm sections were cut and dewaxed, pretreated with a microwave oven in citrate buffer (pH6.0, 10 mM) for 20 min, and incubated in 3% hydrogen peroxide for 15 min to quench endogenous peroxidase. After treating with 10% normal horse serum in phosphate buffered saline (PBS) for 30 min, sections were incubated with anti-PHF24 goat polyclonal antibody (PHF-24 (N-20), 1: 1,000; Santa Cruz, Dallas, TX, USA) for 1 h at room temperature. Primary antibody was detected with VECTASTAIN Elite ABC Kit (Vector Laboratory, Burlingame, CA, USA) and signals were visualized with DAB substrate kit (Nichirei Bioscience Inc., Tokyo, Japan).
For double fluorescence immunohistochemistry, we examined the spinal cords, brain and olfactory bulb fixed with 4% paraformaldehyde and 0.1% glutaraldeyde in 0.1 M PB. Frozen sections were cut at ten-µm and pretreated in 0.3% Triton X-100 in PBS for 15 min. After treating with 10% normal horse serum in PBS for 30 min, sections were incubated overnight at 4°C with the following antibodies: goat polyclonal anti-PHF24 (1:100), rabbit polyclonal anti-calbindin (1:200; Abcam, Cambridge, UK ), mouse monoclonal anti-GABBR2 (1:500; Santa Cruz), mouse monoclonal anti-tyrosine hydroxylase (TH) (1:200; Millipore, Burlington, MA, USA) and mouse monoclonal anti-calretinin (1:200; Leica, Wetzlar, Germany). Then, sections were incubated with secondary antibodies conjugated with Alexa 488 or 568 (1:1,000; Invitrogen, Carlsbad, CA, USA) for 45 min and coverslipped with Fluoro-KEEPER Antifading Reagent (Nacalai Tesque, Inc., Kyoto, Japan). Signals were detected with VS120 Virtual Slide System (OLYMPUS, Tokyo, Japan).
Immunoelectron microscopy
Electron microscopic samples were prepared from the spinal cords and olfactory bulb perfusion-fixed through the left ventricles with 4% PFA and 0.1% glutaraldehyde in 0.1 M PB. After treating with 30% sucrose, samples were frozen at −80°C. Ten-µm frozen sections were cut using a cryostat, rinsed in 0.3% Triton X-100 in PBS 15 min, and treated with 10% normal horse serum in PBS for 30 min. Sections were incubated overnight with goat polyclonal anti-PHF24 (PHF24; 1:1,000) antibody at 4°C overnight, and incubated in 3% hydrogen peroxide for 15 min to quench endogenous peroxidase activity. After washing with PBS, the sections were incubated, sections were treated with VECTASTAIN Elite ABC Kit (Vector Laboratory), treated with 1% glutaraldehyde for 10 min at 4°C, and signals were visualized with DAB substrate kit (Nichirei). Thereafter, sections were post-fixed with 2% osmic acid for 2 h at room temperature, dehydrated and embedded in epoxy resin. Ultrathin sections were cut, and observed using an electron microscope (H-7500, Hitachi, Tokyo, Japan)
Cell counts
The numbers of neuronal marker-positive cells (calbindin, calretinin and TH), PHF24-positive cells and each double positive-cells were counted in the periglomerular layer of the olfactory bulb by microscopic observation. One double fluorescence immunohistochemistry section was obtained from one control animal for each neuronal marker. The sections were scanned by a digital camera (DS-Fi1; Nikon, Tokyo, Japan). Three areas (20× field of view) were randomly selected from one section. The data are presented as the ratios of neuronal marker-positive cells/ PHF24-positive cells and PHF24-positive cells/ Neuronal marker-positive cells. The number of calbindin-positive cells was counted in the lamina II of the thoracic spinal cord and the perigulomerular layer of the olfactory bulb by microscopic observation. Three different sections from three different animals were evaluated in each group. The sections were scanned by a digital camera, and the area of the lamina II and the perigulomerular layer was measured using a software program (WinRoof; Mitani Corp., Fukui, Japan). The data are presented as the number of positive cells/µm2.
Results
PHF24 expression in rat CNS
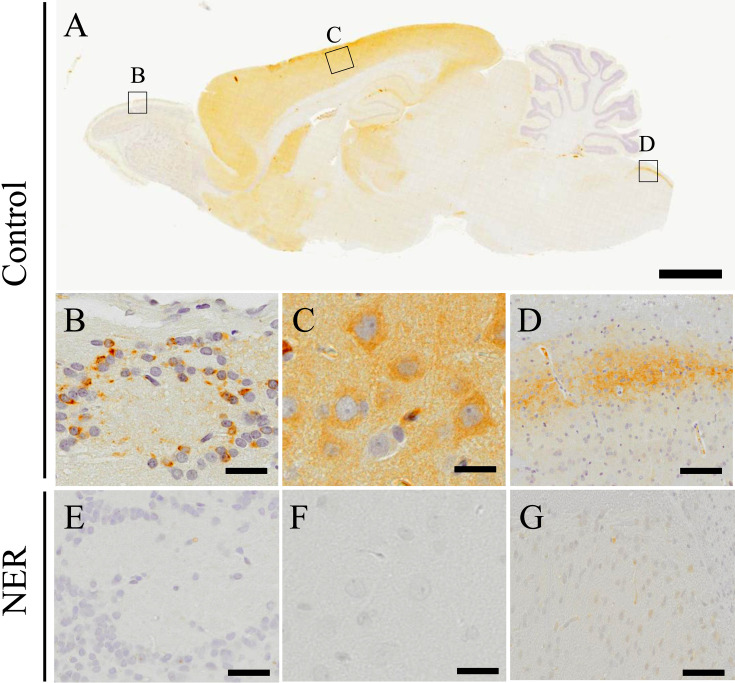
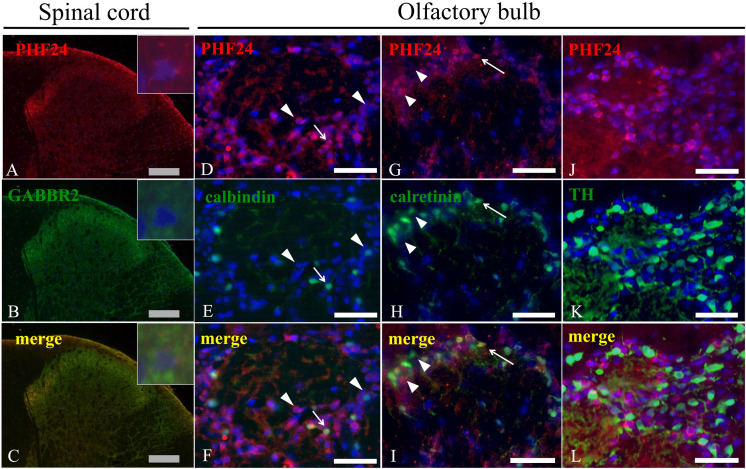
We performed immunohistochemistry for PHF24 in the CNS of the control rats (Figs. 1A–D). Expressions of PHF24 were observed broadly in some regions of the CNS, and characteristic expressions particularly were noted in the olfactory bulb (Fig. 1B), cerebral cortex (Fig. 1C), and medulla oblongata (Fig. 1D). The spinal cords showed the same expression pattern observed in the medulla oblongata (data not shown). While PHF24 was diffusely expressed in the neuropil of the cerebral cortex, particular neurons were stained with PHF24 in the olfactory bulb and spinal cord. In the olfactory bulb, periglomerular cells densely expressed PHF24 (Fig. 1B). The NER demonstrated significant reduction of PHF24 expression in these areas (Figs. 1E–G). PHF24 also diffusely expressed in thalamus, hippocampus, striatum, nucleus accumbens in addition to the cerebral cortex, and the NER showed reduction of PHF24 expression in these areas (Supplementary Fig. 1). Thus, we focused on the PHF24-positive neurons in the olfactory bulb and spinal cords where showed the characteristic expression patterns. It was reported that PHF24 interacts with Gαi of GABAB receptor [7]. Therefore, we performed double immunofluorescence for PHF24 and GABAB receptor2 (GABBR2). PHF24 was strongly expressed in the neurons and fibers of lamina II of gray matter of the spinal cord, and GABBR2 was co-expressed with PHF24 in the neuropil of the spinal cord (Figs. 2A–C). However, PHF24 sometimes expressed independently from GABBR2 at the high magnification (Figs. 2A–C; inset).
Fig. 1.
Immunohistochemistry for PHF24. PHF24 Immunohistochemistry on sagittal section of the CNS in the control rats (A). Black squares indicate the areas of B–D. The expression of PHF24 is mainly observed in the periglomerular layer (B), cerebral cortex (C) and medulla oblongata (D). While PHF24 is expressed broadly in the cerebral cortex (C), the characteristic expression of PHF24 is found in the periglomerular layer (B) and medulla oblongata (C). In the NER, prominent decreased expression is found in the CNS; periglomerular layer (E), cerebral cortex (F) and medulla oblongata (G). Bars: 2 mm (A), 20 µm (B–G).
Fig. 2.
Double immunofluorescence for PHF24 and neuronal markers in the spinal cord and olfactory bulb. PHF24 is expressed in the neuropile of the spinal cord, and the intense expression is observed in the lamina II (A). GABBR2 is also expressed in the same area where PHF24 exhibit (B). Although PHF24 expression pattern is similar to expression of GABBR2 (C), PHF24 sometimes expresses independently from GABBR2 at the higher magnification (Insets). In the olfactory bulb, some of PHF24-positive neurons express calbindin (D–F) and calretinin (G–I). Arrows indicate co-expression (F and I). There are also single positive neurons for calbindin or calretinin as in the olfactory bulb (F and I: arrowheads). On the other hands, PHF24-positive neurons do not show co-expression with TH (J–L). Gray bars: 200 µm, White bars: 40 µm.
Characteristics of PHF24-positive neurons
Periglomerular cells are well-known as interneuron in the olfactory bulb and many interneurons are located in the lamina II of the spinal cord. In order to investigate the property of PHF24-positive neurons, we performed double immunofluorescence for PHF24, calbindin and calretinin, markers for GABAergic interneuron. In the spinal cord, some PHF24-positive neurons co-expressed with calbindin and calretinin (Supplementary Fig. 2). However, there were neurons solely expressed PHF24 with neither calbindin nor calretinin expression.
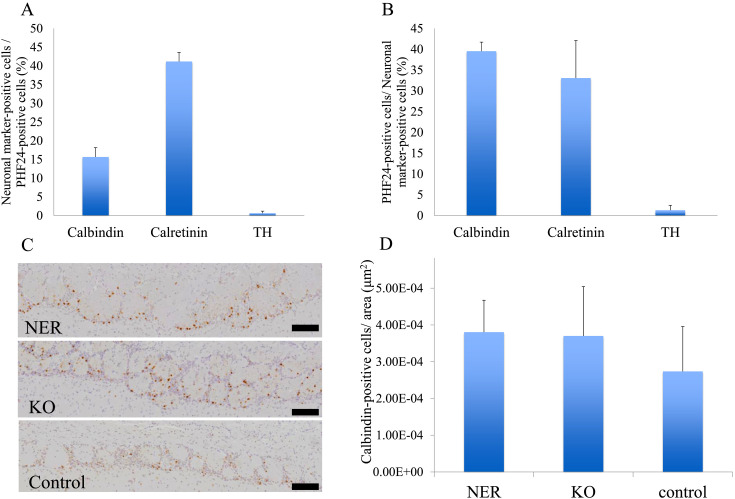
In the periglomerular cells in the olfactory bulb, co-expression of PHF24 and calbindin was observed (Figs. 2D–F). In addition, PHF24-positive neurons expressed calretinin (Figs. 2G–I). On the other hands, almost TH-positive dopaminergic interneurons did not express PHF24 (Figs. 2J–L). The percentages of calbindin, calretinin and TH-positive neurons in PHF24-positive neurons were 15.6, 41.2, and 0.6%, respectively. The percentages of PHF24-positive neurons in calbindin, calretinin and TH-positive neurons were 39.5, 33.1, and 1.2%, respectively (Figs. 3A and B). These results indicated that PHF24 is expressed in the interneurons, especially in the inhibitory interneurons.
Fig. 3.
The positive rates of neuronal markers-positive cells and PHF24-positive neurons and the number of calbindin-positive cells in the olfactory bulb. The percentages of calbindin, calretinin and TH-positive neurons in PHF24-positive neurons in the olfactory bulb were 15.6, 41.2, and 0.6%, respectively (A). The percentages of PHF24-positive neurons in calbindin, calretinin and TH-positive neurons in the olfactory bulb were 39.5, 33.1, and 1.2%, respectively (B) (Three areas in one control. Data are presented as the mean ± SD. (A) SD: 2.52, 2.44, and 0.56 (B) 2.17, 9.04 and 1.17). The number of calbindin-positive interneurons in the olfactory bulb of NER and null rats (C). The calbindin positive cells are counted in the periglomerular layer (D) (n=3 in each group. Data are presented as the mean ± SD. SD: 8.68E-05, 1.64E-04, and 1.22E-04).
Number of calbindin-positive interneurons
We next examined the cell number of calbindin-positive interneurons in the thoracic spinal cord and olfactory bulb. In the spinal cord, there was no significant change in number of calbindin-positive interneurons among NER, PHF24-null and control rats. There was a tendency to increase in a number of calbindin-positive inhibitory interneurons in the olfactory bulb of NER and null rats (Figs. 3C and D). The number of TH-positive interneurons did not show any difference in the spinal cord and olfactory bulb among NER, null and control rats (data not shown).
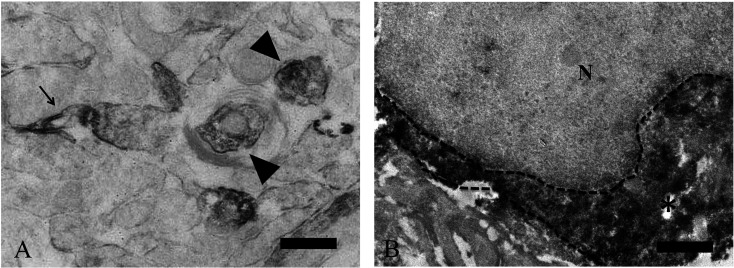
Immunoelectron microscopy for PHF24
Immunoelectron microscopy revealed that strong PHF24 signals were found in the presynaptic terminal (Fig. 4A; arrowheads) and synaptic membrane in the neuropil of olfactory bulb and spinal cord (Fig. 4A; arrow). In addition, PHF24 signals were diffusely distributed in the cytoplasm of the periglomerular cells (Fig. 4B). Cytoplasmic expression of PHF24 did not coincide with any organelle. Our data indicated that PHF24 is located in the cytosol of soma and presynaptic terminal.
Fig. 4.
Immunoelectron microscopy for PHF24 in the spinal cord and olfactory bulb. Expression of PHF24 located in presynaptic terminals (A: arrowheads) and synaptic membrane (A: arrow). This finding is observed both in the spinal cord and olfactory bulb and a representative example in the spinal cord is presented (A). In the olfactory bulb, positive reaction for PHF24 is diffusely found in the neuronal cytoplasm (B: asterisk). N: Nucleus Broken line: a border of cytoplasm Black bars: 100 µm (A), 1 µm (A, B).
Discussion
In rat CNS, some area showed the expressions of PHF24. The cerebral cortex, hippocampus, striatum, thalamus and nucleus accumbens exhibited diffuse expressions of PHF24 that included cytoplasm of neurons and the neuropile. In the medulla oblongata and spinal cord, the strong expressions of PHF24 were limited in the lamina II. In the olfactory bulb, the particular neurons expressed PHF24. In the cerebral cortex and others that expressed PHF24 diffusely, a lot of neurons was positive for PHF24 and it was hard to recognized the preference of PHF24-positive neurons. Therefore, we focused on the spinal cord and olfactory bulbs where PHF24-positive neurons were more easily identified. As a result, our data demonstrated PHF24 was expressed in the cytoplasm and synapses of the inhibitory interneurons. We consider that these differences in expression patterns of PHF24 were attributed to numbers of PHF24-positive neurons and the amount of synapses. All of these expressions were markedly reduced in the NER.
In rat olfactory bulb, periglomerular cells are entirely divided into three subsets, which are respectively positive for calbindin, calretinin, and TH [11]. Calcium-binding protein including calbindin and calretinin is mainly expressed in GABAergic neurons. TH is known as the marker for dopaminergic neuron. Our double immunohistochemistry data indicated that PHF24 was expresseed in GABAergic interneurons that were positive for calbindin or calretinin. Calbindin and calretinin are mainly expressed in the lamina II in rat spinal cord [12], and PHF24-positive cells were found in the same area. This distribution suggests that PHF24 may play important roles in the population of the inhibitory interneurons.
In NER, the number of calbindin-positive interneurons in the thoracic spinal cord was not changed compared with control rats. It may suggest that lack of PHF24 doesn’t affect a morphological development of calbindin-positive interneurons. On the other hand, there was a tendency to increase in the number of inhibitory interneurons in the olfactory bulb in NER. TH-positive interneuron did not show any increasing tendency in their number in NER. Neurogenesis in the olfactory bulb is well known even in adulthood and the area of subventricular zone continually provides periglomerular interneuron [13]. There is a possibility that dysfunction of GABAergic interneurons caused by lack of PHF24 expression may stimulate neurogenesis of calbindin-positive cell, but not TH-positive cell in the NER and PHF24-null rats.
Our present study demonstrated diffuse PHF24-expression in the lamina II of spinal cord. Many GABAergic synapses also exist in this region, and our immunoelectron microscopic data showed PHF24 was expressed in the presynaptic structures. In the olfactory bulb, most GABAB receptors expressed in the periglomerular layers and mainly located at the presynaptic membrane [14]. Our data suggests diffuse PHF24-expression may coincide with GABAergic synapses. Kuramoto et al. reported that PHF24-expression was also found in the cerebral cortex and hippocampus, and its expression was diffusely distributed in the neuropile [6]. Therefore, those diffuse expressions of PHF24 in the CNS may relate to the amount of GABAB receptors in those regions.
Many GABAB receptors are located at pre- and post-synaptic terminals and inhibit synaptic release. Loss of PHF24 may cause a dysfunction of GABAB signaling followed by hyperexcitability of interneurons. It has been already reported that abnormal behavior of interneuron and loss of GABAergic synapse in the entorhinal cortex leads to temporal lobe epilepsy [15]. In addition, Bekenstein et al. reported that dormancy of inhibitory interneuron in hippocampus CA1 occurred in a model of temporal lobe epilepsy [16], and it is assumed that this dormant inhibitory interneuron is related to significant disturbances of pre- and post-synaptic GABAB-receptor-mediated processes in the region CA1 in a chronic model of temporal lobe epilepsy induced by continuous hippocampal stimulation [17]. Thus in the NER, it is thought that deficiency of PHF24 induces incomplete GABAB signaling, and these functional disorders would be related to epileptogenesis in the NER. In addition, PHF24-null rats show high sensitivity for chemically-induced seizures [10]. PHF24 may role as the inhibitory modulator for epilepsy.
There is a close relation between olfactory bulb and temporal lobe [18]. The olfactory axon crosses the lamina propria and reaches the glomerulus of the olfactory bulb where signals are integrated. In the glomeruli, the axons of the olfactory sensory neurons form synapses with the dendrites of the mitral and the tufted cells. The axons originated in the mitral and tufted cells leave the olfactory bulb and project to the piriform lobe and to the limbic system [19]. Thus, the olfactory bulb is thought be related to the temporal lobe epilepsy. Intense olfactory input induced by octanol elicits seizures in the transgenic mice engineered to express an octanal receptor in almost all olfactory sensory neurons [20]. In addition, olfactory kindling can cause epilepsy in normal rats [21].
In consideration of high sensitivity of PHF24-null rats for epilepsy [10], In NER, lack of PHF24 may induce functional disorder of inhibitory interneurons in the glomeruli. It is likely that insufficiently modulated olfactory input plays as a kindling, and repeated kindling from the olfactory bulb induced hyperexcitability in the limbic system, leading to epilepsy.
Supplementary Material
Acknowledgments
We are thankful to the National BioResource Project-Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing the NER and PHF24-null rats.
References
- 1.Noda A, Hashizume R, Maihara T, Tomizawa Y, Ito Y, Inoue M, et al. NER rat strain: a new type of genetic model in epilepsy research. Epilepsia. 1998; 39: 99–107. doi: 10.1111/j.1528-1157.1998.tb01281.x [DOI] [PubMed] [Google Scholar]
- 2.Ohno Y, Shimizu S, Harada Y, Morishita M, Ishihara S, Kumafuji K, et al. Regional expression of Fos-like immunoreactivity following seizures in Noda epileptic rat (NER). Epilepsy Res. 2009; 87: 70–76. doi: 10.1016/j.eplepsyres.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 3.Takeda A, Iida M, Ando M, Nakamura M, Tamano H, Oku N. Enhanced susceptibility to spontaneous seizures of noda epileptic rats by loss of synaptic zn(2+). PLoS One. 2013; 8: e71372. doi: 10.1371/journal.pone.0071372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiura Y, Hanaya R, Serikawa T, Kurisu K, Sakai N, Sasa M. Involvement of Ca(2+) channels in abnormal excitability of hippocampal CA3 pyramidal cells in noda epileptic rats. J Pharmacol Sci. 2003; 91: 137–144. doi: 10.1254/jphs.91.137 [DOI] [PubMed] [Google Scholar]
- 5.Maihara T, Noda A, Yamazoe H, Voigt B, Kitada K, Serikawa T. Chromosomal mapping of genes for epilepsy in NER: a rat strain with tonic-clonic seizures. Epilepsia. 2000; 41: 941–949. doi: 10.1111/j.1528-1157.2000.tb00276.x [DOI] [PubMed] [Google Scholar]
- 6.Kuramoto T, Voigt B, Nakanishi S, Kitada K, Nakamura T, Wakamatsu K, et al. Identification of candidate genes for generalized tonic-clonic seizures in noda epileptic rat. Behav Genet. 2017; 47: 609–619. doi: 10.1007/s10519-017-9870-2 [DOI] [PubMed] [Google Scholar]
- 7.Gaillard S, Lo Re L, Mantilleri A, Hepp R, Urien L, Malapert P, et al. GINIP, a Gαi-interacting protein, functions as a key modulator of peripheral GABAB receptor-mediated analgesia. Neuron. 2014; 84: 123–136. doi: 10.1016/j.neuron.2014.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003; 424: 775–778. doi: 10.1038/nature01859 [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Wang F, Fischer G, Hogan QH, Yu H. Peripheral nerve injury induces loss of nociceptive neuron-specific Gαi-interacting protein in neuropathic pain rat. Mol Pain. 2016; 12: 1744806916646380. doi: 10.1177/1744806916646380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serikawa T, Kunisawa N, Shimizu S, Kato M, Alves Iha H, Kinboshi M, et al. Increased seizure sensitivity, emotional defects and cognitive impairment in PHD finger protein 24 (Phf24)-null rats. Behav Brain Res. 2019; 369: 111922. doi: 10.1016/j.bbr.2019.111922 [DOI] [PubMed] [Google Scholar]
- 11.Rogers JH. Immunohistochemical markers in rat brain: colocalization of calretinin and calbindin-D28k with tyrosine hydroxylase. Brain Res. 1992; 587: 203–210. doi: 10.1016/0006-8993(92)90998-O [DOI] [PubMed] [Google Scholar]
- 12.Ren K, Ruda MA. A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Res Brain Res Rev. 1994; 19: 163–179. doi: 10.1016/0165-0173(94)90010-8 [DOI] [PubMed] [Google Scholar]
- 13.Figueres-Oñate M, López-Mascaraque L. Adult olfactory bulb interneuron phenotypes identified by targeting embryonic and postnatal neural progenitors front. Front Neurosci. 2016; 10: 194. doi: 10.3389/fnins.2016.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panzanelli P, López-Bendito G, Luján R, Sassoé-Pognetto M. Localization and developmental expression of GABA(B) receptors in the rat olfactory bulb. J Neurocytol. 2004; 33: 87–99. doi: 10.1023/B:NEUR.0000029650.28943.b2 [DOI] [PubMed] [Google Scholar]
- 15.Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006; 26: 4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993; 259: 97–100. doi: 10.1126/science.8093417 [DOI] [PubMed] [Google Scholar]
- 17.Mangan PS, Lothman EW. Profound disturbances of pre- and postsynaptic GABAB-receptor-mediated processes in region CA1 in a chronic model of temporal lobe epilepsy. J Neurophysiol. 1996; 76: 1282–1296. doi: 10.1152/jn.1996.76.2.1282 [DOI] [PubMed] [Google Scholar]
- 18.Restrepo D, Hellier JL, Salcedo E. Complex metabolically demanding sensory processing in the olfactory system: implications for epilepsy. Epilepsy Behav. 2014; 38: 37–42. doi: 10.1016/j.yebeh.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fioretti AB, Fusetti M, Eibenstein A. The Predictive Role of Hyposmia in Alzheimer’s Disease. 2011. pp. 259–278. In: The Clinical Spectrum of Alzheimer’s Disease -The Charge Toward Comprehensive Diagnostic and Therapeutic Strategies. (de la Monte S.M. eds.), INTECH, Rijeka. [Google Scholar]
- 20.Nguyen MQ, Ryba NJ. A smell that causes seizure. PLoS One. 2012; 7: e41899. doi: 10.1371/journal.pone.0041899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara A, Watanabe Y, Takechi K, Ishikawa T, Kaida Y, Akagi M, et al. The usefulness of olfactory bulb kindling as a model for evaluation of antiepileptics. Epilepsia. 2010; 51: 445–453. doi: 10.1111/j.1528-1167.2009.02378.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.