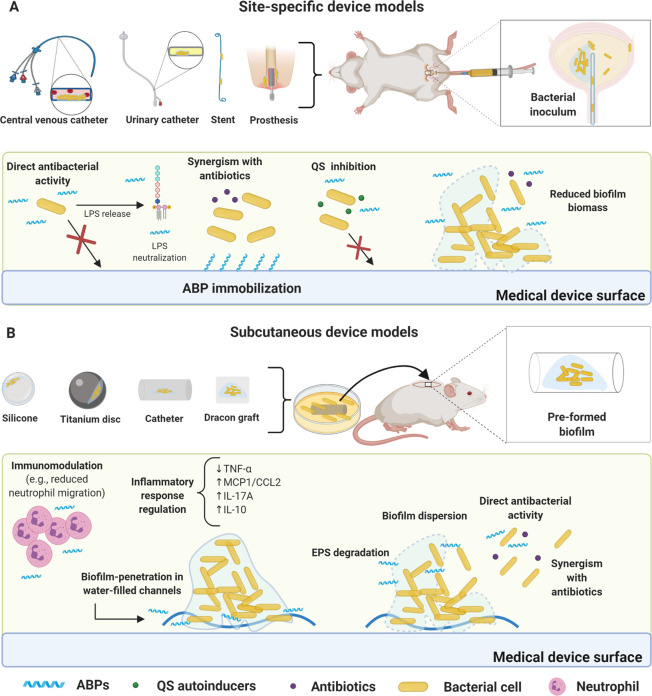
Figure 2.
Foreign body murine models for biofilm infections and proposed ABPs modes of action. (A) Site-specific device model. The most common devices used to assess ABPs activity include catheter and urinary stents, central venous catheters (CVC), and periprosthetic implants, all surgically inserted. ABPs can be immobilized on the device’s surface, thus inhibiting biofilm formation for extended periods. This strategy can be used in association with conventional antibiotics. Some ABPs also inhibit bacterial communication (e.g., QS). Direct bacterial activity is also a common mechanism (e.g., ABPs alone or in synergism with antibiotics) for inhibiting biofilm formation. In preformed biofilms, ABPs can reduce biofilm biomass. Furthermore, direct bacterial activity is also a common mechanism. (B) Subcutaneous device models. In these models, biofilm is usually previously formed in the device (e.g., titanium disc, silicone beads, Dracon graft, and catheter). The infected device is inserted subcutaneously in the animal (mouse or rat), followed by ABP treatment. ABPs have demonstrated direct antibacterial activity in most cases with bacterial membrane disruption. The antibiofilm activity has also been achieved via EPS degradation, leading to biofilm dispersion and enabling synergism with antibiotics. Additionally, ABPs have been shown to penetrate the biofilms through water channels and disperse biofilm cells, followed by direct antibacterial effects. Immunomodulatory responses have also been observed, including reduced neutrophil migration and cytokine regulation (e.g., TNF-α, MCP1/CCL2 IL-17A, and IL-10). All figures were made by the authors with a subscription version of BioRender.com.