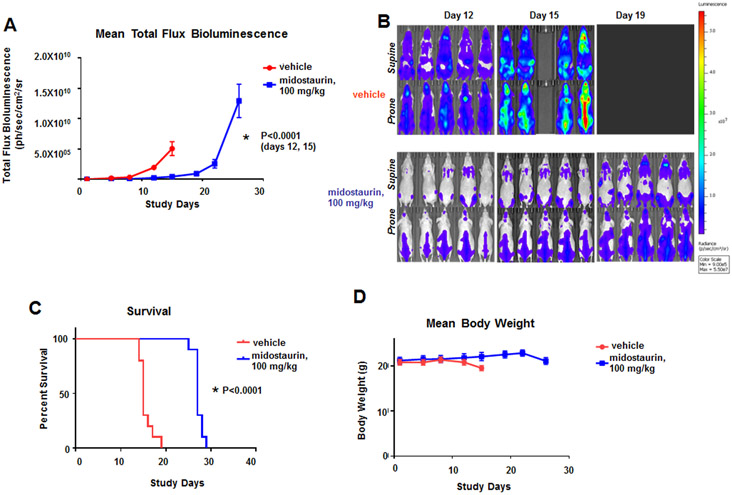
Figure 6. Effects of midostaurin in vivo against mice harboring Ba/F3.FLT3.CBL.Y371H-luc+ cells.
(A) Measure of leukemia burden in vehicle- versus midostaurin-treated mice for CBL.Y371H murine model. Vehicle-treated, female Ncr nude mice were administered vehicle by oral gavage once daily x 21 days. Midostaurin-treated mice were administered 100 mg/kg midostaurin by oral gavage once daily x 21 days. For total flux bioluminescence, the Mann-Whitney test (two-tailed) was carried out for CBL.Y371H BLI for Day 12: Vehicle versus midostaurin, p=0.0001. The Mann-Whitney test (two-tailed) was carried out for CBL.Y371H BLI for Day 15: Vehicle versus midostaurin. P<0.0001. (B) Effects of midostaurin in vivo against mice harboring Ba/F3.FLT3.CBL.Y371H-luc+ cells. Supine and Prone (High Scale), Day 12 – 19 Representative Images (n=5). (C) Measure of survival of vehicle- versus midostaurin-treated mice for CBL.Y371H murine model. Gehan-Breslow-Wilcoxon test was carried out for survival curve comparisons. P<0.0001. (D) Mean weights of mice from the CBL.Y371H murine model.