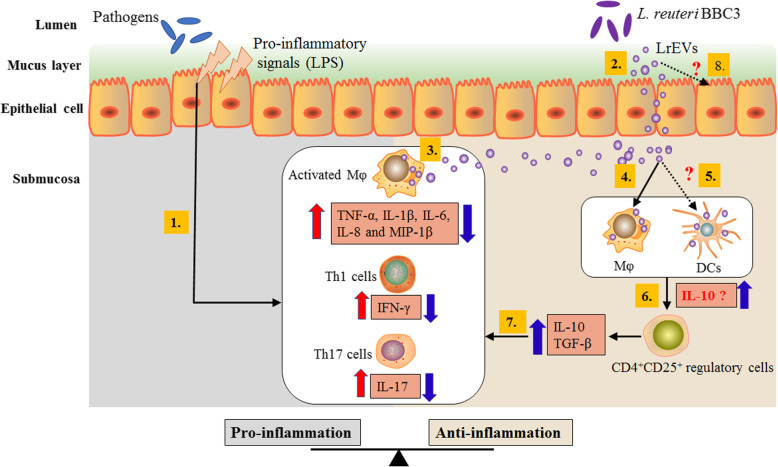
Fig. 8.
Proposed mechanism of LrEVs-mediated bacteria-host crosstalk to drive the intestinal immune homeostasis against pathogens-induced inflammation in the chicken model. In inflammatory bowel disease, pathogens remarkably proliferate in the gut lumen and produce pro-inflammatory signals, such as lipopolysaccharide (LPS), which activate inflammatory cells, including macrophages (Mφ), Th1 and Th17 cells, to produce pro-inflammatory responses (1) [57]. L. reuteri BBC3 releases nanosized and highly biocompatible EVs that can drive the long-distance transport of interior molecules throughout the intracellular compartments in a concentrated, protected and targeted manner (2) [72]. These vesicles can suppress the pro-inflammatory mediators produced by inflammatory cells (activated Mφ) (3), and activate innate immune cells, including naïve Mφ (4) and possibly dendritic cells (DCs) (5), to produce immunoregulatory cytokines (possibly including IL-10) (6) that induce the development of immunoregulatory CD4+CD25+ cells. These resulting CD4+CD25+ cells can produce anti-inflammatory cytokines IL-10 and TGF-β that inhibit the production of pro-inflammatory cytokines (7) [62]. Further studies are required to investigate the potential interactions between LrEVs and other intestinal immune cells, especially DCs (5) and epithelial cells (8)