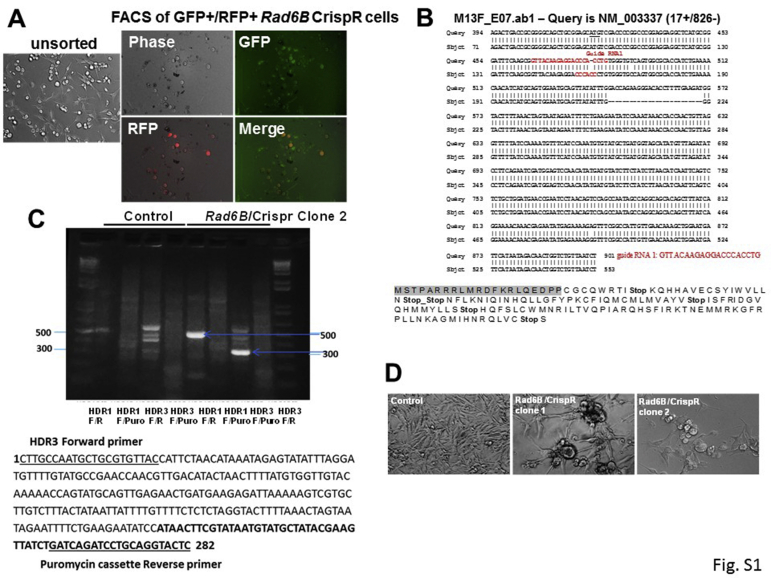
Supplemental Figure S1.
Characterization of M14 RAD6B knockout clones produced by CrispR/Cas9 strategy. A: Green fluorescent protein (GFP)+ (expressing guide RNA/Cas9) and red fluorescent protein (RFP)+ (indicative of homology-directed vector integration) cells were isolated by fluorescence-activated cell sorting (FACS) analysis. B: RAD6B knockout clone 1, resulting from guide RNA 1, mediated Cas9 cleavage, and the guide RNA 1 sequence is indicated in red. Full-length RAD6B cDNA was amplified, and sequence was aligned with human RAD6B mRNA (https://www.ncbi.nlm.nih.gov/nucleotide; accession number NM_003337). The effect of Cas9 gene editing on the translated product is shown below. The gray shaded sequence represents the amino terminal end of the normal Rad6B amino acid sequence. C: RAD6B loss in clone 2 was screened by PCR analysis of genomic DNA using forward (F) and reverse (R) primers specific for homology-directed repair (HDR) vectors that are selective for guide RNA 1 (HDR1) or guide RNA 3 (HDR3), and a reverse primer within the puromycin integration cassette (Puro) inserted in the HDR vectors. Note amplification of 282-bp band with HDR3 forward and Puro reverse primers in RAD6B knockout clone 2, indicating RAD6B gene disruption by puromycin cassette integration. Below, sequence analysis of clone 2 for integration of RFP-puromycin cassette. The positions of HDR3 forward and Puro reverse primers used for PCR amplification are shown as underlined sequences. The bolded sequence indicates integrated puromycin cassette. D: Phase contrast microscopy of the RAD6B knockout clones. Note spheroid cluster formation in the knockout clones compared with control cells with mesenchymal phenotype.