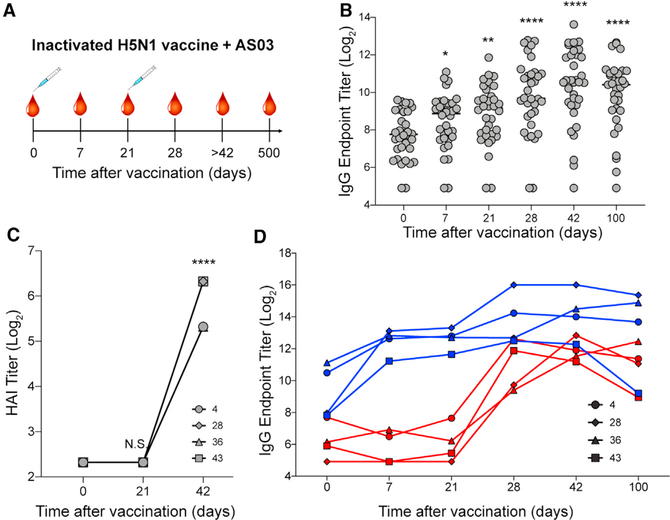
Figure 1. Novel H5N1 vaccination elicits robust IgG response to stem and head of hemagglutinin (HA).
(A) H5N1 vaccine trial: 34 healthy adults received the first immunization of inactivated H5N1 (A/Indonesia/5/2005) vaccine adjuvanted with AS03 at day 0 and the second immunization at day 21. Blood samples were collected on days 0, 7, 21, 28, 42 or 100, and 500 after vaccination.
(B) ELISA binding titers of serum IgG to recombinant H5 HA during the course of vaccination.
(C) HAI titers of serum IgG to recombinant H5 HA at days 0, 21, and 42 from subjects 4, 28, 36, and 43.
(D) ELISA binding titers of serum IgG to the head or stem domains of H5 HA using H5 head-specific (red) and stem-specific (blue) probes from subjects 4, 28, 36, and 43.
p values were determined by an unpaired t test: *p < 0.05, **p < 0.01, ****p < 0.0001. N.S., not significant. See also Figure S1.