FIGURE 3.
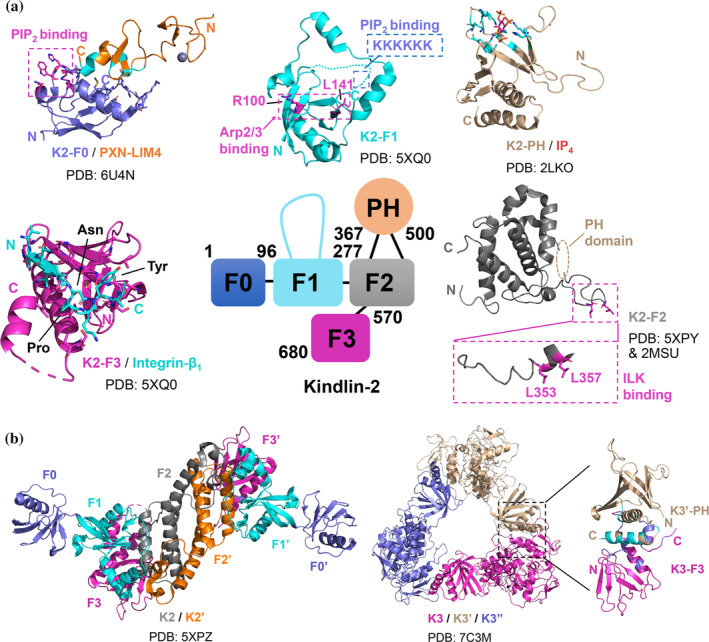
The binding interfaces of kindlin‐mediated interactions in integrin and FA signaling. (a) Domain organization of kindlin‐2 and reported structures of individual domains of kindlin‐2 in apo or complex form. The binding interfaces/sites for target proteins are indicated in the figure. Important residues involved in the binding interfaces or targeted in mutagenesis studies are shown in stick representation. The interface residues of paxillin LIM4 (within a cut‐off of 5 Å) that interact with kindlin‐2 F0 are colored in cyan. The interface residues of kindlin‐2 PH (within a cut‐off of 5 Å) that interact with IP4 are colored in cyan. (b) Reported crystal structures of truncated kindlin‐2 and full‐length kindlin‐3 in respective dimeric and trimeric conformation. The interface residues of kindlin‐3 F3 and kindlin‐3’ PH (within a cut‐off of 5 Å) are colored in blue and cyan, respectively. PXN, paxillin. K2, kindlin‐2. K3, kindlin‐3. N and C indicate the N‐terminus and C‐terminus of the protein molecule, respectively. PDB codes used to generate the figures are indicated
