Figure 1.
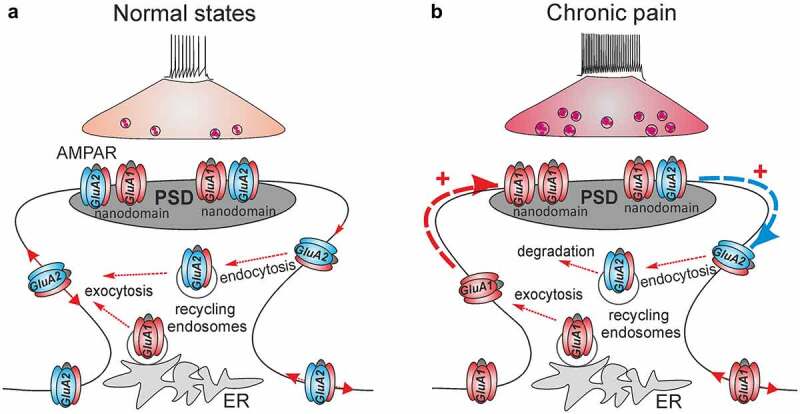
Spinal AMPAR trafficking for nociceptive signaling in normal (healthy) and chronic pain states. (a) After exocytosis from the intracellular stores such as the endoplasmic reticulum (ER), AMPAR laterally diffuse across the extrasynaptic plasma membrane to and from the postsynaptic density (PSD), undergoing constituent endocytosis/exocytosis cycles via recycling endosomes until are immobilized within the nanodomains of the receptors at PSD. (b) In chronic pain, the long-lasting activation of nociceptive afferents triggers the mobilization of the GluA2-containing AMPAR at PSD and the receptors undergo internalization from the synapses (blue arrow) followed by degradation (endocytosis via recycling endosomes). The GluA1-containing AMPAR replenish a pool of surface receptors and are mobilized to synapses (red arrow) to replace the GluA2-containing AMPAR within the nanodomains at PSD. Such a re-arrangement of the spinal AMPAR of different subunit compositions represents nociceptive plasticity in central pain pathways for the chronic pain maintenance
