Figure 3.
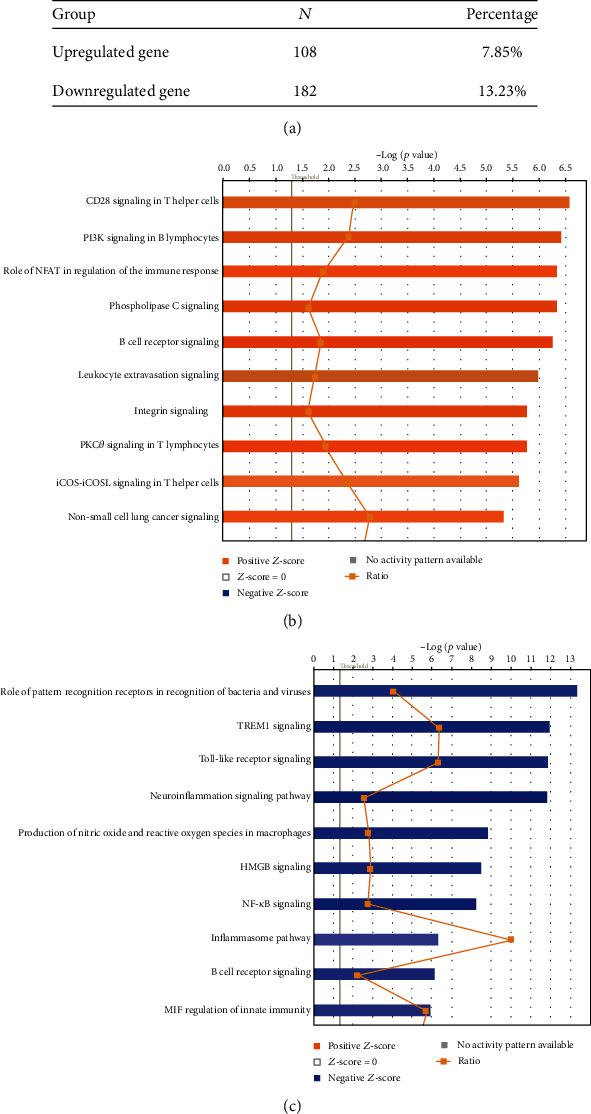
(a) Low-intensity ultrasound (LIUS) upregulated 108 out of 1376 (7.9%) innate immunome genes and downregulated 182 out of 1376 (13.2%) innate immunomic genes in rat bone marrow cells (GSE70662), suggesting that LIUS suppresses innate immunomic gene expressions more than it increases them in bone marrow cells. The LIUS-modulated genes are listed in Supplemental Table 3. (b) LIUS upregulated 108 genes that were significantly involved in 54 signaling pathways in bone marrow cells. The top ten pathways include CD28 signaling in T cells, PI3K signaling in B lymphocytes, the role of NFAT in regulation of immune responses, phospholipase C signaling, B cell receptor signaling, leukocyte extravasation signaling, integrin signaling, PKC zeta signaling in T lymphocytes, ICOS-ICOSL signaling in T helper cells, and non-small-cell lung cancer signaling. (c) LIUS downregulated 182 genes that were significantly involved in 70 signaling pathways in bone marrow cells. The top ten pathways include the role of pattern recognition receptors, TREM1 signaling, Toll-like receptor signaling, neuroinflammation signaling, production of nitric oxide and reactive oxygen species (ROS) in macrophages, HMGB1 signaling, NF-κB signaling, inflammation pathway, B cell receptor signaling, and MIF regulation of innate immunity. These results suggest that LIUS inhibits numerous key innate immunity and inflammation pathways in bone marrow cells, which may be responsible for LIUS therapeutic effects of inflammation suppression since nine out of the top ten pathways are related to danger signal recognition and inflammation initiation.
