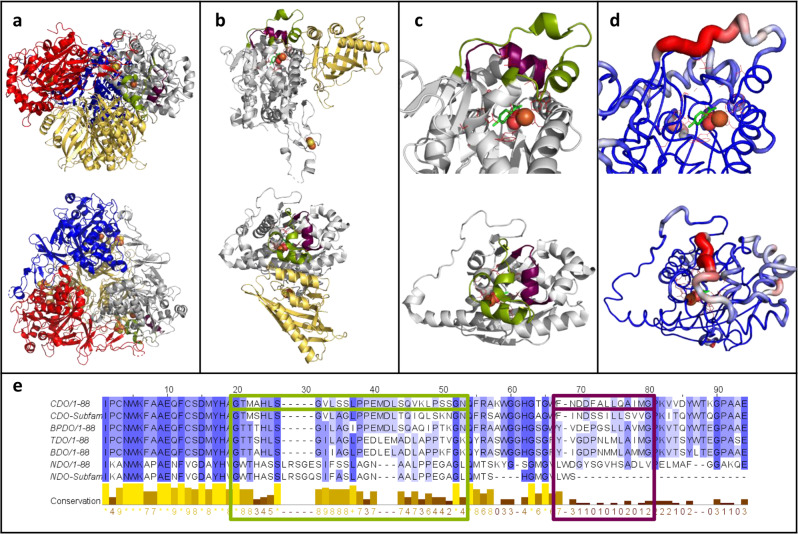
Fig. 1. The selected loops 1 and 2 of the cumene dioxygenase from Pseudomonas fluorescens IP01 (PDB entry: 1WQL).
a Side view (top structure) and top view (bottom structure) of the hexameric quaternary structure oxygenase consisting of three α-subunits (gray, blue and red) and three β-subunit (yellow). Loop 1 (29 residues, green), loop 2 (13 residues, purple), as well as the catalytic iron (orange sphere) and the Rieske center (yellow and orange spheres) are shown. b Side view and top view of the dimeric structure of the α-subunits (gray) and the corresponding β-subunit (yellow) with loop 1, loop 2, catalytic iron and Rieske cluster in the same color code like 1a. c Side view and top view of an enlargement of loop 1 (green) and loop 2 (purple) in the crystal structure of one α-subunit (gray) of the oxygenase of the CDO. The catalytically active iron (orange sphere), molecular oxygen (red spheres) and active-site residues (red sticks) are shown, as well as the docked substrate (R)-limonene (green sticks). d B-factor indicated by color-coded cartoon of the crystal structure from low (blue) to high (red) from the same angles like c. Iron, molecular oxygen, (R)-limonene and active-site residues are shown in the same color pattern as in 1a. e Sequence alignment of the α-subunit of the oxygenases from CDO, the CDO subfamily, biphenyl dioxygenase (BPDO) from Paraburkholderia xenovorans (LB400), toluene dioxygenase (TDO) from Pseudomonas putida F1, benzene dioxygenase (BDO) from Pseudomonas putida ML2, naphthalene dioxygenase (NDO) from Pseudomonas sp. NCIB 9816-4 and the NDO subfamily. The degree of conservation is shown from low (white) to high (blue), as well as in the conservation line. Loop 1 (green), loop 2 (purple), and the corresponding residues in the other ROs are highlighted by frames in the corresponding colors.