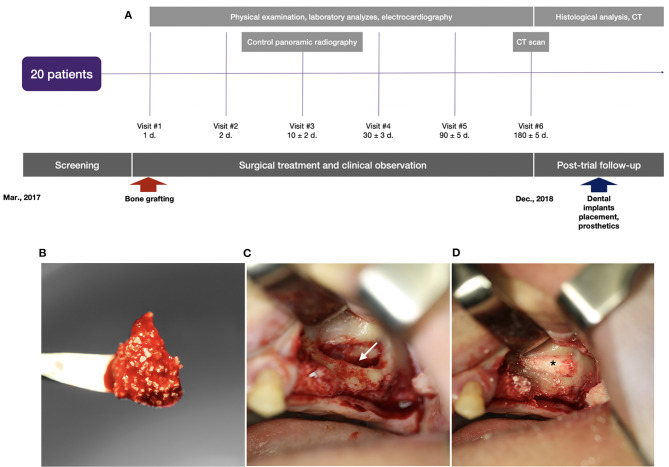
Figure 5.
Clinical trial with illustration of the typical indication for bone grafting: (A) scheme of the clinical trial design, (B) granules of the gene-activated bone substitute in the blood clot before implantation into the bone defect; (C) the space (indicated by the white arrow) opened to be filled in by the gene-activated bone substitute in the sinus-lifting procedure; (D) granules of the implanted gene-activated bone substitute (indicated by the asterisk) within the bone cavity.