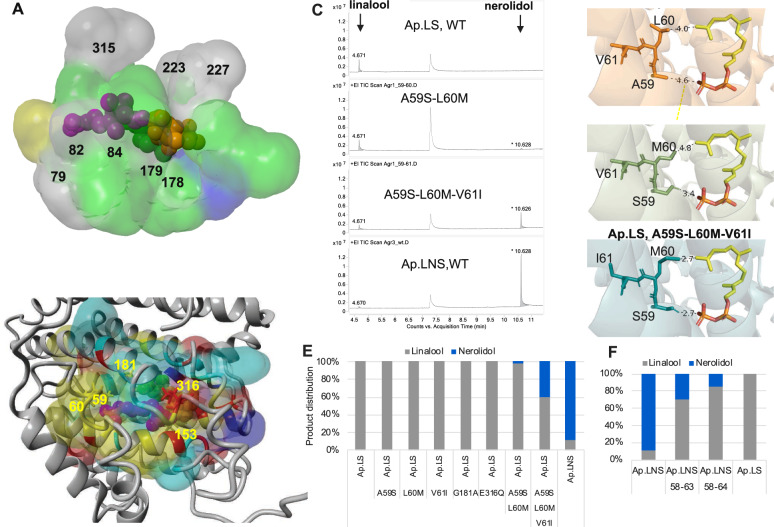
Fig. 7. Structure analysis for the understanding of activity and specificity.
A The aligned 3D active site surfaces of Ap.LS, Sc.LNS, and Ma.LS. The residues are listed in Supplementary Table S4. Gray: conserved residues among the linalool synthases of three kingdoms; green: not conserved in plant linalool synthase; blue: not conserved in bacterial linalool synthase; yellow: not conserved in fungal linalool synthase. B Highlight of the key residue difference between Ap.LS and Ap.LNS. Detailed comparison of binding-pocket residues in Supplementary Table S5. C Mutation of the key residues responsible for Ap.LS specificity. D The interactions of the selected residues with FPP in Ap.LS wild type (WT) and its mutants (distance by dash line, unit: Å). E Quantitative comparison of Ap.LS and its mutants. F Quantitative comparison of Ap.LNS and its mutants.