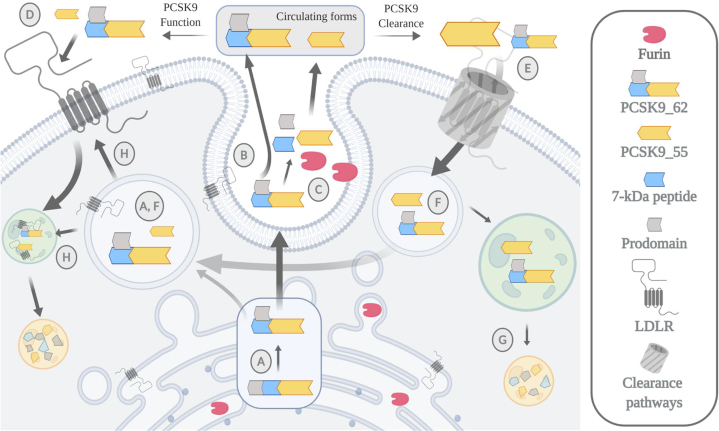
Fig. 6.
Working model. Newly synthesized PCSK9 undergoes an autocatalytic cleavage inside the cell that releases the prodomain (13 kDa) from the peptide chain. (A) The cleaved prodomain binds back to the main protein through noncovalent forces generating a heterodimer of 62 + 13 kDa, representing the mature form of PCSK9 (PCSK9_62). (B) This is an essential step for the proper secretion of PCSK9_62 into circulation. PCSK9_62 can also undergo a second cleavage, in the extracellular space, mediated by the protease furin. (C) Furin cleaves PCSK9 at the N-terminal region releasing an ∼7 kDa peptide and potentially the prodomain, generating the second most common form of plasma PCSK9 with a size of 55 kDa (PCSK9_55). (D) Once in circulation, both forms of PCSK9 induce hepatic LDLR degradation, though PCSK9_62 is more active than PCSK9_55. (E) PCSK9 elimination from circulation is mediated by LDLR-dependent and LDLR-independent pathways. Potentially, the furin-cleaved form of PCSK9, PCSK9_55, has a shorter half-life owing to a faster clearance rate than PCSK9_62. (F) Once inside the cell, PCSK9_55 cannot get secreted back to circulation and (G) before going through catabolism, (H) the intracellular pool of PCSK9_55 is capable of inducing LDLR degradation though less so relative to PCSK9_62.