Figure 1.
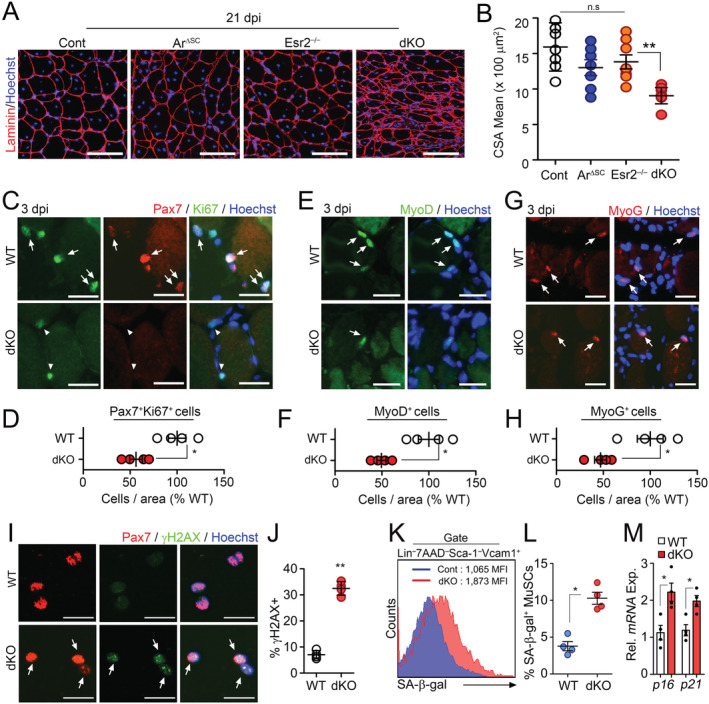
Accelerated aging phenotypes of muscle stem cells (MuSCs) in the absence of intrinsic sex steroid receptor signalling. (A–H) Three‐month‐old WT (Con), Pax7 CreER ;Ar f/y (ArΔSC), Esr2 −/− and Pax7 CreER ;Ar f/y ;Esr2 −/− (dKO) mice were orally administered with tamoxifen (Tmx) for five consecutive days. Tibialis anterior (TA) muscles were injured by BaCl2 injection at 6 months after Tmx administration. TA muscles were analysed at 21 days post‐ BaCl2 injury (dpi). Immunohistochemical (IHC) staining for laminin (A) and quantification of mean cross‐section‐area (CSA) of regenerating TA muscles (B). Representative IHC images and quantification of Pax7 and Ki67 costaining (C and D) in TA muscles at 3 dpi. Arrows and arrowheads indicate Pax7+Ki67+ and Pax7+Ki67− cells, respectively. Representative images and quantification of MyoD (E and F) and Myogenin (MyoG, G and H) staining in TA muscles at 3 dpi. Arrows indicate MyoD + or MyoG + cells. (I) Immunostaining and (J) quantification for Pax7 and γH2AX in freshly isolated MuSCs from uninjured mice. Arrows indicate Pax7+γH2AX+ cells. (K) Flow cytometry analysis of SA‐β‐Gal activity, and (L) quantification of SA‐β‐Gal+ cells in isolated MuSCs. (M) Relative mRNA expression of senescence‐associated genes in MuSCs. Scales: 100 (A), 50 (C, E, and G), and 20 μm (I). Comparisons by one‐way ANOVA with Tukey's post hoc test (B), unpaired t‐test (D, F, H, J, and M), and Mann–Whitney U test (L). Bars, mean ± SEM. A, B; n = 7 TA muscles from 4 animals per group and C–M; 4 animals per group *P < 0.05, **P < 0.01. The detailed procedure of CSA quantification applied throughout this study is described in the supporting information.
