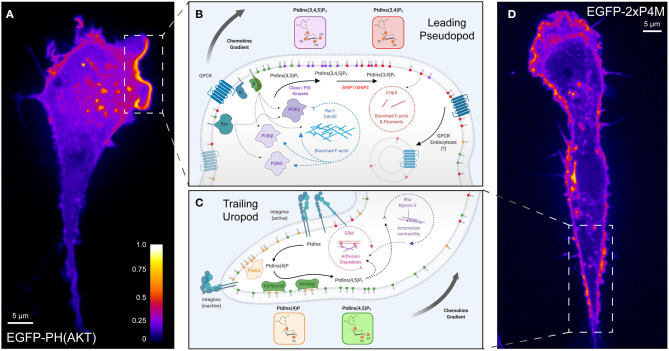
Figure 2.
Polarization of phosphoinositide signals during chemotaxis. (A) A representative confocal micrograph of PH-AKT expressed in a RAW264.7 monocytic cell undergoing chemokinesis (random motion) in the presence of growth factors from fetal-bovine serum. PH-AKT bi-specifically recognizes PtdIns(3,4,5)P3 and PtdIns(3,4)P2. The image has been pseudocolored to reflect the abundance of the probe. (B) Phosphoinositide signaling controlling “frontness” during leukocyte chemotaxis. A signal arising from the stimulation of cell surface GPCRs and associated activation of G-proteins (β, γ) by chemokines lead to the activation of class I PI3Ks to produce PtdIns(3,4,5)P3 from PtdIns(4,5)P2. Together with PtdIns(3,4)P2, the product of dephosphorylation of PtdIns(3,4,5)P3, 3-phosphoinositides in the leading-edge membrane mediate a feed-forward loop that activates Rho- and Arf-family GTPases and Lmpd to drive actin polymerization and locally amplify PI3K-signaling. (C) Engagement of substratum by leukocytes favors the “backness” signals PtdIns(4)P and PtdIns(4,5)P2. PI4KA mediates the synthesis of PtdIns(4)P from PtdIns, which is necessary for the activation of PIP5KIγ90 and the generation of PtdIns(4,5)P2. RhoA signaling networks and ERM proteins regulated by PtdIns(4,5)P2 are critical for leukocyte rolling adhesion, diapedesis, and uropod contraction during migration. (D) A representative confocal micrograph of 2xP4M expressed in a RAW264.7 cell undergoing chemokinesis (random motion) in the presence of growth factors from fetal-bovine serum. The P4M domain derived from Legionella's SidM specifically recognizes PtdIns(4)P. The image has been pseudocolored to reflect the abundance of the probe in the Golgi, endolysosomes, and the plasma membrane including in the region of the uropod. GPCR, G protein-coupled receptor; PI3K, phosphoinositide 3-kinase; SHIP, SH2 domain-containing inositol polyphosphate 5-phosphatase; Lmpd, Lamellipodin; ERM, Ezrin-Radixin-Moesin.