Figure 1. Hazard Ratios (HRs) by Day Postrandomization.
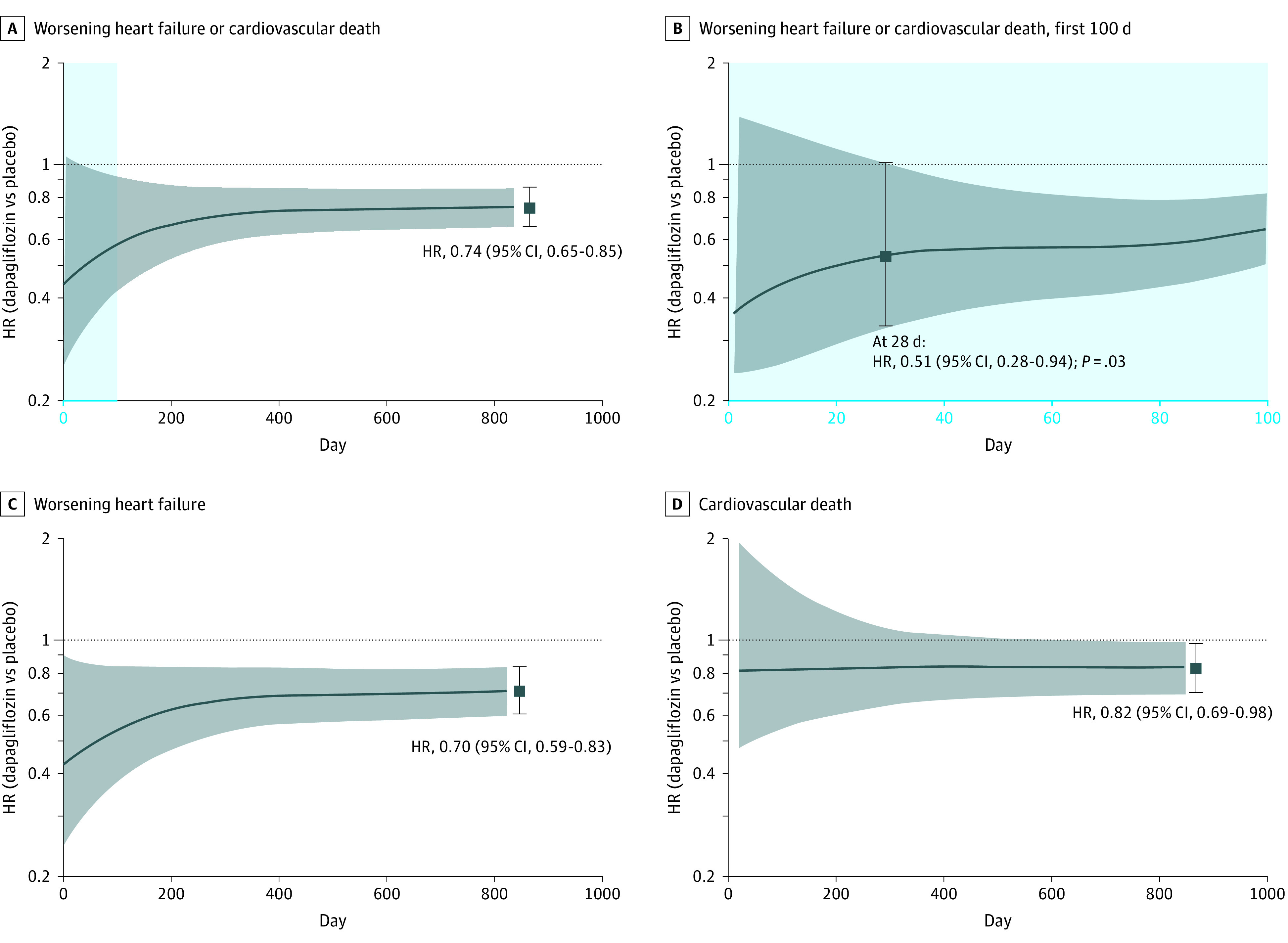
A, Dapagliflozin vs placebo for the primary efficacy outcome; B, dapagliflozin vs placebo for the primary efficacy outcome in the first 100 days; C and D, the individual components of worsening heart failure (C) and cardiovascular death (D). The HRs and 95% CIs observed at the end of the trial for each outcome are provided as a point of reference.1 A reduction in the risk of the primary efficacy outcome of cardiovascular death or worsening heart failure event was statistically significant by 4 weeks after randomization (HR at 28 days, 0.51 [95% CI, 0.28-0.94]; P = .03).
