Abstract
Aims:
This study investigated the impact of promoter methylation of flouropyrimidine (FP) metabolizing and cyclooxygenase 2 (COX2) genes on their mRNA expression and on the clinical outcome of colorectal cancer (CRC) patients.
Methods:
Methylation specific-PCR and real time-PCR of thymidylate synthase (TS), thymidine phosphorylase (TP), dihydropyrimidine dehydrogenase (DPD) and COX2 were performed at baseline and after 3 and 6 months of FP therapy. Pairwise comparisons were conducted between the subgroups of CRC patients. The event free survival (EFS) and the hazard of progression were estimated by univariate and multivariate analyses.
Results:
At baseline CRC patients, both TS and TP were overexpressed, in spite of the unmethylation of TS and the full methylation of TP genes. Significant downexpression of DPD and COX2 were associated their promoter’s methylation. At the end of FP therapy, TS, DPD and COX2 were overexpressed by 7.52, 2.88 and 3.45 folds, respectively, while TP was downexpressed by 0.54 fold. However, no change was observed in the methylation status of genes with FP therapy. Pairwise comparisons revealed significant difference in the expression and the methylation status of genes according to the clinicopathological characters of CRC patients either at baseline or after FP therapy. The overexpression of DPD and COX2 genes were indicators for a poor EFS of CRC patients. Also, the high level of COX2 expression was found to be significantly correlated with the hazard of progression (HR = 1.73, 95% CI = 1.02-3.03).
Conclusion:
The promoter methylation of FP metabolizing and COX2 genes has significant impact on the expression and the treatment outcome of CRC patients.
Keywords: Colorectal cancer, fluoropyrimidine metabolizing, cyclooxygenase 2, promoter methylation, gene expression, survival and progression
Introduction
Colorectal cancer (CRC) is from the most commonly diagnosed cancer in the world, and accounts for 8% of all cancer related deaths. There is a remarkable increase in the burden of CRC in developing countries that are witnessing an economic advancement, adoption of a Western life style and of dietary habits characterised by higher intake of meat, fat and total calories, along with increasing life expectancy and population growth.1 Based on the Volgestein model, multiple reversible and irreversible factors accumulate and participate in CRC development. Certain genetic and epigenetic factors along with the chronic inflammatory condition of the colorectum are found to be combined in the pathogenesis of CRC disease.2 Treatment with fluoropyrimidine (FP) – based therapy is still the treatment of choice in CRC, in spite of the primary and the acquired resistances frequently observed.3
Epigenetics have a prominent role in the carcinogenesis of CRC. DNA methylation in the promoters of tumour suppressor gene, which is known to be enriched with CpG dinucleotides, induces transcriptional silencing. Recently, these hypermethylated sites have been included as potential biomarkers in early risk evaluation, diagnosis and prognosis of CRC disease.4 In addition to the impact of global DNA methylation on the treatment outcome of CRC disease,5 also localised or site specific DNA methylation at the promoters of drug metabolizing genes and drug transporters have a role in the chemosensitivity of cancer patients to certain treatment.6 That role of epigenetics in the response to treatment is called pharmaco-epigenetics, and it has been emerged as an important element in personalised medicine, and also it is the clue for the inter-individual variability to treatment. Because of the reversibility of the epigenetic modifications, hence, their levels should be monitored in the affected tissue, and in the body fluids.7
The effect of epigenetics on the expression of FP metabolizing enzymes (thymidylate synthase (TS), thymidine phosphorylase (TP) and dihydropyrimidine dehydrogenase (DPD)) is found to be from the predisposing causes to the variation in the patients’ response to FP therapy.8 TS, the rate-limiting enzyme of DNA synthesis, is a valuable target for many antimetabolite drugs such as 5-fluorouracil (5-FU), and its level of expression is proportional with the response of CRC patients to 5-FU therapy.9 TS expression was found to be epigenetically regulated by microRNA-215.10 This microRNA controls the sensitivity of colon cancer cells to TS inhibitor drugs through increasing the G2/M cell cycle check point and reducing the proliferation of cells.10
TP enzyme is involved in the metabolic activation of 5-FU selectively in CRC cells. It has also counter angiogenic and chemotactic effects, which confuses its usefulness, and suggests for the epigenetic role in such effects.11 A transcriptional silencing of TP gene was shown in mesothelioma as a result of the DNA methylation of extracellular growth factor-1, modulating the efficacy of antifolate therapy.12 Moreover, DPD deficiency was found to be correlated with the degree of DPD promoter methylation, inducing toxicity to FP therapy and causing progression of CRC disease.13
So, this study investigated the contribution of the promoter methylation of FP metabolizing (TS, TP and DPD) genes to therapeutic effectiveness of FP based therapy in CRC patients. In addition, the DNA methylation of inflammatory marker (cyclooxygenase 2; COX2) is measured as a determinant for the chemosensitivity of CRC patents because of the significant correlation between the level of COX2 expression and the level of its promoter methylation in early and advanced CRC tissues.14,15
In this study, genes promoters’ methylation and expression were measured at baseline and after 3 and 6 months of FP therapy, in the peripheral mononucleated lymphocytes of 43 CRC patients, stratified into subgroups according to their clinicopathological characters. The impact of methylation and mRNA expression of genes was correlated with the event free survival (EFS) and the hazard of progression of patients after 3 years of follow up.
Patients and Methods
Sample collection and lymphocytic cell pellet preparation
This prospective study included 43 patients with confirmed diagnosis of CRC, who were enrolled to the National Cancer Institute, Cairo University during the period from February 2014 to December 2014. At baseline, peripheral whole blood samples were collected from the 43 CRC patients. After 3 and 6 months during their course of FP therapy, another 43 and 32 blood samples were collected from the patients. The median age of our CRC patients was 45 years old, and slight male predominance was exhibited among them. Almost the two-thirds of our patients were non-smokers, with normal level of tumour markers (CEA and CA19.9). Most of our patients received combined FP based therapy of capecitabine or 5-FU with oxaliplatin, Table 3.
Table 3.
The clinicopathological characters of CRC patients.
| Count | % | |
|---|---|---|
| Total | 43 | 100 |
| Age | ||
| ⩽45 y | 22 | 51.1 |
| >45 y | 21 | 48.8 |
| Sex | ||
| Female | 19 | 44.2 |
| Male | 24 | 55.8 |
| Smoking | ||
| Non smoker | 28 | 65.1 |
| Smoker | 15 | 34.9 |
| Baseline CEA | ||
| Normal | 30 | 69.8 |
| High | 13 | 30.2 |
| Baseline CA19.9 | ||
| Normal | 28 | 65.1 |
| High | 15 | 34.9 |
| Site of tumour | ||
| Non specified | 5 | 11.6 |
| Right colon | 11 | 25.6 |
| Left colon | 10 | 23.3 |
| Rectum | 17 | 39.5 |
| Pathology | ||
| Adenocarcinoma | 33 | 76.7 |
| Mucinous | 10 | 23.3 |
| T | ||
| T2 | 6 | 14.0 |
| T3 | 26 | 60.5 |
| T4 | 11 | 25.6 |
| N | ||
| Negative | 27 | 62.8 |
| Positive | 16 | 37.2 |
| M | ||
| Negative | 23 | 53.5 |
| Positive | 20 | 46.5 |
| Stage | ||
| II | 7 | 16.3 |
| III | 16 | 37.2 |
| IV | 20 | 46.5 |
| First line FP therapy | ||
| Single (Capecitabine) | 7 | 16.3 |
| Combined (+Oxaliplatin) | 36 | 83.7 |
Data presented as count and percentage of 43 CRC patients at different clinicopathological subgroups.
Abbreviations: CEA, carcinoembyonic antigen; CA19.9, carbohydrate antigen 19.9; T, tumour burden; N, lymph node; M, metastasis.
Whole blood samples were also collected from 32 matched healthy controls in sex and age (male: female ratio = 1:1.3 and age = 38 ± 15.2, P = .89). Mononucleated lymphocytic cell pellets were isolated as described previously in Fouad et al.5
Methylation specific-PCR (MS-PCR)
DNA was extracted from patients’ lymphocytic cell pellets using DNA isolation and purification kit supplied from Jena Bioscience (Germany). Genomic DNA (500 ng) was bisulfite modified by (EZ DNA MethylationTM Kit (Zymoresearch, Germany). MS-PCR was carried out following the method of Herman et al.16 PCR was performed using primer pairs which target one or more CpG of the core promoters in the sense strand of the 4 genes (TS, TP, DPD and COX2). Two sets of primers for each gene were designed by Meth prime design tool. The primers that amplify sequences in which CpGs are methylated (M-primers), and the primers that amplify sequences in which CpGs are unmethylated (U-primers) were purchased from Invitrogen by ThermoFisher Scientific, UK Table 1. In 25 µl, PCR amplification was carried out using host start Taq DNA polymerase master mix (Zymo research, Germany), bisulfite-modified DNA template (2 µl) and 10 µM of the forward and reverse primers. Amplification conditions was adjusted according to manufacturer recommendations and genes’ annealing temperatures optimised, as seen in Table 1. Control PCRs lacking genomic DNA were performed for each set of reactions. PCR reaction products were loaded onto horizontal electrophoresis on agarose gels, stained with ethidium bromide and visualised under UV illumination.
Table 1.
MS-PCR primers.
| Genes | Primers | Sequence | Tm |
|---|---|---|---|
| TS | M-Forward primer | AAGGCGCGGTCGATTAGAC | 53.2 |
| Sequence ID: NG_028255.1 | M-Reverse primer | AAAACCACGAATATAACACAA AAC G | |
| Region: 4784. . . .4818 | U-Forward primer | GGT TTA GAG AAG GTG TGG TTGATTAGAT | 57 |
| U-Reverse primer | CACAAAACCACAAATATAACACAAAAC A | ||
| TP | M-Forward primer | TTAGCGTTGCGTCGCGTT C | 53.2 |
| Sequence ID: NG_011860.1 | M-Reverse primer | CCGACCAATCCCCCGATA C | |
| Region: 3816. . . .3825 | U-Forward primer | TGG GAT TTTAGTGTTGTGTTGTGTTT | 53.2 |
| U-Reverse primer | CCCAACCAATCCCCCAATAC | ||
| DPD | M-Forward primer | GGTTGTCGTGTTTGGCGC | 52.6 |
| Sequence ID: NG_008807.2 | M-Reverse primer | ATC TAC CAA TAA CAA ACC CTCCTTACG | |
| Region: 132138. . .132151 | U-Forward primer | GTTGTGGTTGTTGTGTTTGGTGT | 53.5 |
| U-Reverse primer | ATCTACCAATAACAAACCCTCCTTACA | ||
| COX2 | M-Forward primer | TTAGATACGGCGGCGGCGGC | 60 |
| Sequence ID: NG_028206.2 | M-Reverse primer | TCTTTACCCGAACGCTTCCG | |
| Region: 4536..4549 | U-Forward primer | ATAGATTAGATATGGTGGTGGTGGT | 60 |
| U-Reverse primer | CACAATCTTTACCCAAACACTTCCA |
Real time-PCR (RT-PCR)
Total RNA was extracted from cell pellets with total RNA purification kit (Direct-Zol RNA Kit, Zymo Research, Germany). Complement DNA synthesis step was performed according to the manufacturer’s instructions using Revert Aid First Strand cDNA synthesis kit (ThermoFisher, UK). Quantitative PCR was done according to the manufacturer’s instructions using Syber Green PCR Master Mix (Applied Biosystems, USA). Reverse and forward sequences of primer genes (TS, TP, DPD and COX2) were designed by NCBI-NIH tool, obtained from Invitrogen by ThermoFisher Scientific, UK, as summarised in Table 2. For every patient, genes CT values were normalised to the CT value of the housekeeping gene (B-actin); in order to calculate 2−∆Ct. Also, genes CT values were normalised to the CT value of genes expression at the baseline; in order to calculate the fold change after the FP therapy (2−∆∆Ct).
Table 2.
RT-PCR primers.
| TS | Forward primer | TACCTGGGGCAGATCCAACA |
| Sequence ID: NM_001071.4 | Reverse primer | AGAGGGAATTCATCTCTCAGGC |
| Region: 187. . . 256 | ||
| TP | Forward primer | TGGACAAGCATTCCACAGGG |
| Sequence ID: NM_001257989.1 | Reverse primer | CGCTGATCATTGGCACCTTG |
| Region: 530. . . . 733 | ||
| DPD | Forward primer | GGACAGAGTCCAGCTACTGTG |
| Sequence ID: XM_017000510.1 | Reverse primer | TGCGCTGTTCCAGATAAGGT |
| Region: 2685 to 2708 | ||
| COX2 | Forward primer | CAGCACTTCACGCATCAGTT |
| Sequence ID: NM_000963.4 | Reverse primer | TCTGGTCAATGGAAGCCTGT |
| Region: 698. . . 717 | ||
| B-actin | Forward primer | CCAGAGCAAGAGAGGTATCC |
| Sequence ID: NM_001100.3 | Reverse primer | CTGTGGTGGTGAAGCTGTAG |
| Region: 286 to 304 |
Statistical analysis
IBM SPSS statistical package version 24 was used in data manipulation. Numeric data explored for normality using Kolmogrov-Smirnov test and Shapiro-Wilk test. Categorical data were expressed as numbers and percentages, while numerical data were summarised as medians and interquartile ranges (IQR). Patients were stratified into subgroups according to their clinicopathological and molecular features. Pairwise comparison between subgroups of CRC patients was tested by Chi-Square test for the categorical data, while Mann-Whitney for 2 groups of numerical one, and Kruskal Wallis for more than 2 groups of numerical data. The effect of FP therapy on genes expression over time (3 and 6 months) in certain subgroup of CRC patients was tested by Wilcoxon matched test. After 3 years of follow up, EFS of patients were tested by Kaplan-Meier procedure. EFS was calculated from the date of resection or neoadjuvant therapy to the date of recurrence, progression or death, which occurred first. EFS for patients who neither progressed, relapsed, nor died, was censored at last assessment prior to loss of follow-up. Significant clinicopathological and molecular variables on patients’ survival were tested for their hazardous effects on progression using multivariate COX proportion hazard model. All P-values were two-sided, adjusted for multiple comparisons, and the P-values ⩽.05 were considered significant.
Results
Patients’ characteristics
As shown in Table 3, the median age of our CRC patients was 45 years old. 55.8% of them were males, and 65.1% were non-smokers. Normal CEA and CA19.9 tumour marker levels were recorded in 69.8% and 65.1% of patients, respectively. Twenty-one patients were colon cancer patients, while 17 of them were rectal cancer. 76.7% of our patients were with adenocarcinoma and 74.5% were with T2 and T3 tumours. Negative lymph nodes were detected in 27 patients, and 23 patients were with negative metastasis and at stage II and III of the disease. Out of 43 patients, 7 patients received single agent of FP based therapy (capecitabine) and 36 patients had combined FP therapy (with oxaliplatin).
Baseline unmethylation with upregulation of TS gene, and the increase of TS expression with FP therapy
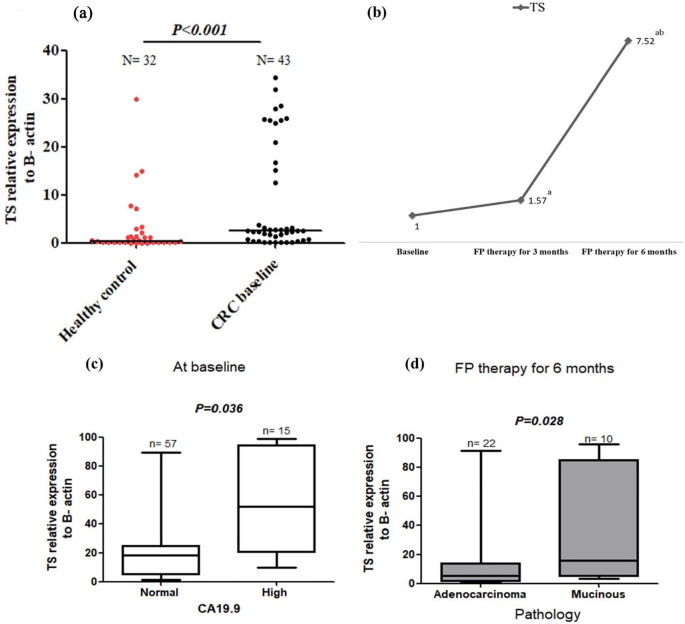
TS was unmethylated (UTS) in 90.7% of CRC patients, and the unmethylated status has not been changed after FP treatment, Table 4 and Figure 1. The mRNA expression of TS was significantly higher in chemonaïve CRC patients compared with healthy controls (median = 31.08 vs 15.15 folds, P = .014), Figure 2a. TS expression was significantly increased by 1.57 folds after 3 months of FP therapy, and furtherly increased by 7.52 folds after 6 months therapy, Figure 2b. At baseline, TS expression was significantly higher in patients with high baseline CA19.9 level (median TS expression = 6.56 folds) compared with patients with normal baseline CA19.9 (median TS expression = 2.87 folds), Figure 2c. After 6 months of FP therapy, TS expression was significantly higher in patients with mucinous tumours compare with patients with adenocarcinoma type of tumours (P = .028), Figure 2d. Univariate and multivariate survival analyses revealed insignificant difference in patients’ EFS time and the hazard of progression at low and high expression levels of TS Figures 6 and 7.
Table 4.
The association of the promoter methylation of fluoropyrimidine metabolising genes with their expression level in CRC patients.
| TS promoter methylation form | % of baseline CRC patients |
|||
|---|---|---|---|---|
| Total (N = 43) (%) | TS baseline median expression level (%) |
P-value | ||
| ⩽2.98 | >2.98 | |||
| UTS | 90.7 | 42.0 | 36.0 | .793 |
| UMTS | 2.3.0 | 2.0 | 0.0 | NA |
| MTS | 7.0 | 0.0 | 6.0 | NA |
| TP promoter methylation form | Total (N = 43) (%) | TP baseline median expression level (%) |
P-Value | |
| ⩽6.92 | >6.92 | |||
| UTP | 0.0 | 0.0 | 0.0 | NA |
| UMTP | 0.0 | 0.0 | 0.0 | NA |
| MTP | 100 | 47.1 | 40.8 | NA |
| DPD promoter methylation form | Total (N = 43) (%) | DPD baseline median expression level (%) |
P-Value | |
| ⩽4.26 | >4.26 | |||
| UDPD | 0.0 | 0.0 | 0.0 | NA |
| UMDPD | 4.7 | 3.9 | 0.0 | NA |
| MDPD | 95.3 | 58.8 | 24.5 | <.001 |
| COX2 promoter methylation form | Total (N = 43) (%) | COX2 baseline median expression level (%) |
P-Value | |
| ⩽3.95 | >3.95 | |||
| UCOX2 | 4.7 | 2.0 | 2.0 | NA |
| UMCOX2 | 39.5 | 25.5 | 8.2 | .006 |
| MCOX2 | 55.8 | 35.3 | 12.2 | .002 |
Data presented as the percentage of patients at different promoter gene methylation forms (U, UM and M) and different levels of expression. The median baseline expression levels of TS, TP, DPD and COX2 in 43 CRC patients were 2.98, 6.92, 4.26 and 3.95 were. Significant P-values marked with bold italic font.
Abbreviations: U, unmethylated; UM, partial methylated; M, methylated; TS, thymidylate synthase; TP, thymidine phosphorylase; DPD, dihydropyrimidine dehydrogenase; COX2, cyclooxygenase 2; NA, not applicable.
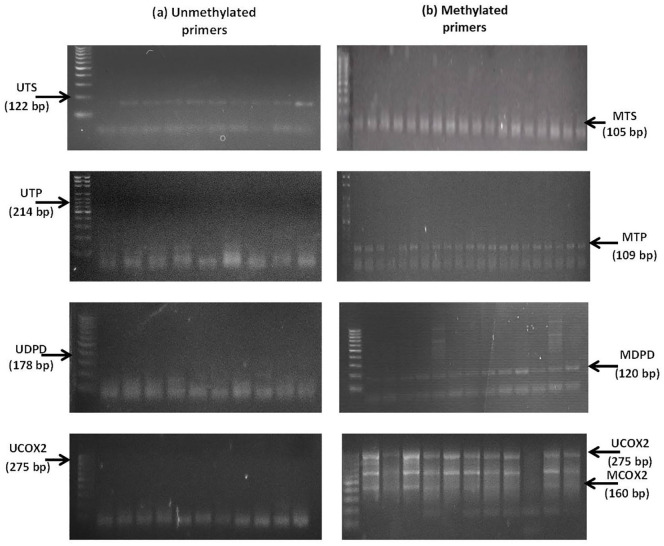
Figure 1.
TS, TP, DPD and COX2 genes methylation forms (U, UM and M) for selected number of 43 CRC patients. Each 3 consecutive bands after the DNA ladder represent the gene expression before and after 3 and 6 months of FP therapy for each CRC patient: (a) the unmethylated genes primers in the left side (UTS, UTP, UDPD and UCOX2) and (b) the methylated genes primers in the right side (MTS, MTP, MDPD and MCOX2).
Abbreviations: U, unmethylated; UM, partial methylated; M, methylated genes.
DNA extracted from patients at baseline and after 3 and 6 months of FP therapy were bisulphite converted, and then amplified with the methylated and un-methylated primers of the target genes. PCR products were horizontally electrophoresed and imaged on UV-transilluminator system.
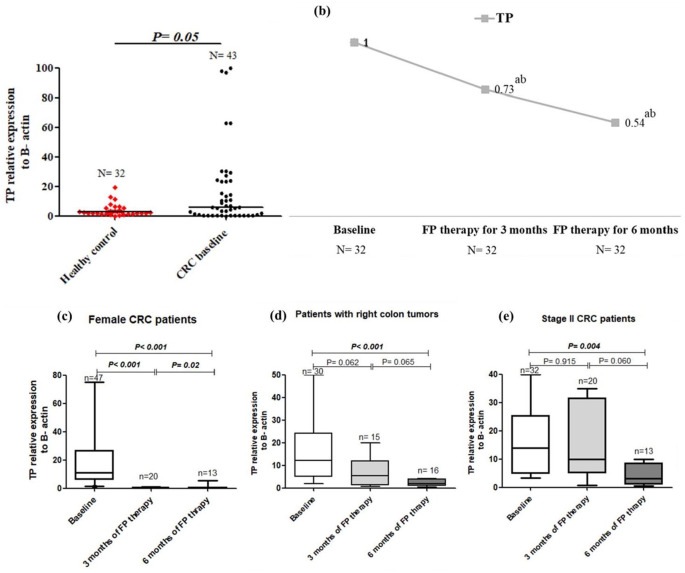
Figure 2.
TS expression in healthy control and baseline CRC patients (a), the change in TS expression after 3 and 6 months of FP therapy normalised to their baseline levels (b), TS expression at low and high CA19.9 subgroups of baseline CRC patients (c) and TS expression in adenocarcinoma and mucinous pathological subtypes of tumours after 6 months of FP therapy (d).
aIs a significant difference when CRC patients after 3 and 6 months of FP therapy were compared with their baseline level, P value ⩽.05.
bIs a significant difference when CRC patients after 6 months of FP therapy were compared with their level after 3 months of FP therapy, P value ⩽.05. Total RNA was extracted from the isolated lymphocytic cell pellets of healthy control and CRC patients at baseline and after 3 and 6 months of FP therapy. RNA was converted into cDNA, and then RT-PCR amplification was conducted with the designed TS gene sequence.
Figure 6.
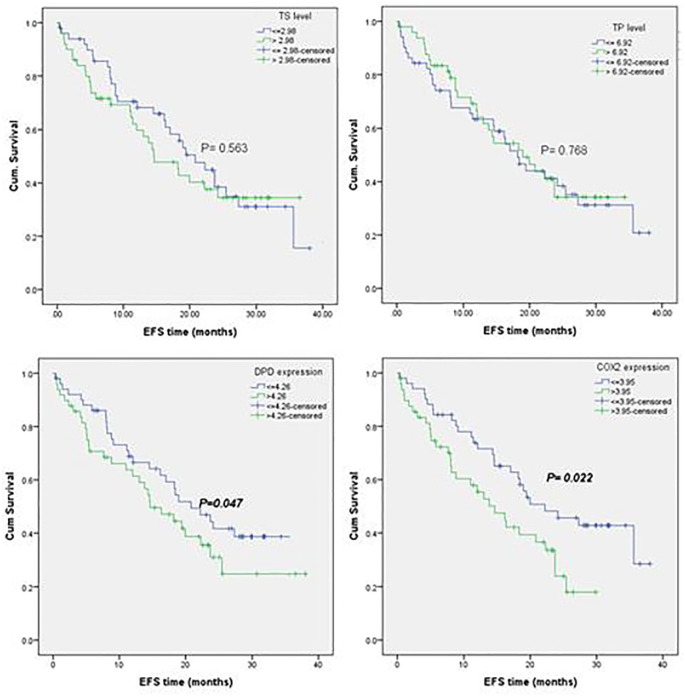
Kaplan-Meier EFS curves for molecular subgroups of CRC patients. TS ⩽ 2.98 and TS > 2.98 (a), TP ⩽ 6.92 and TP > 6.92 (b), DPD ⩽ 4.26 and DPD > 4.26 (c) and COX2 ⩽ 3.95 and COX2 > 3.95 (d).
The baseline median relative expression of TS, TP, DPD and COX2 genes normalised to β-actin for 43 CRC patients were 2.98, 6.92, 4.26 and 3.95, respectively. Patients were stratified around the medians calculated for the 4 genes’ baseline expression levels, and then the time to recurrence or progression was calculated with Kaplan-Meier test of survival.
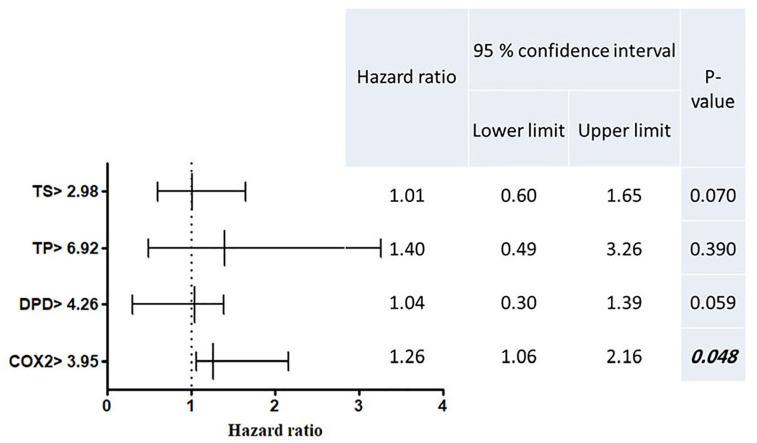
Figure 7.
Multivariate COX regression model for the hazard ratio of recurrence or progression and its 95% confidence interval for molecular subgroups of CRC patients.
Significant P-value ⩽.05 marked bold italic font, and indicates significant hazard of recurrence or progression during the EFS time. Patients were stratified around the medians calculated for the 4 genes’ baseline expression levels, where the baseline median expression of TS, TP, DPD and COX2 genes were 2.98, 6.92, 4.26 and 3.95, respectively. Then the hazard ratio of table variables (genes expression > baseline median level), were compared with the hazard ratio of reference variables (genes expression ⩽ the baseline median level which equal 1), by multivariate COX regression test of survival.
Baseline, full methylation but upregulation of TP gene, and the decrease in TP expression with FP therapy
The examined sequence of TP promoter showed full methylation (MTP) in 100% of CRC patients either at baseline or after FP therapy, Table 4 and Figure 1. The mRNA expression of TP was significantly higher at baseline CRC patients than in healthy controls (median = 22.52 vs 5.52 folds, P = .002), Figure 3a. FP therapy significantly decreased the expression of TP to 0.73 and 0.54 folds after 3 and 6 months of therapy respectively, Figure 3b. The decrease in TP level over FP therapy was significantly observed in female patients (P = .048), patients with right colon tumours (P = .016) and stage II patients (P = .045), Figure 3c, d and e, respectively. The median EFS times and hazard of progression were not changed significantly between the different expression levels of TP, Figures 6 and 7.
Figure 3.
TP expression in healthy control and baseline CRC patients (a), the change in TP expression after 3 and 6 months of FP therapy normalised to their baseline levels (b), TP expression in female patients over time (c), TP expression in patients with right sided tumours (d) and TP expression in stage II CRC patients (e).
aIs a significant difference when CRC patients after 3 and 6 months of FP therapy were compared with their baseline level, P value ⩽.05.
bIs a significant difference when CRC patients after 6 months of FP therapy were compared with their level after 3 months of FP therapy, P value ⩽.05. Total RNA was extracted from the isolated lymphocytic cell pellets of healthy control and CRC patients at baseline and after 3 and 6 months of FP therapy. RNA was converted into cDNA, and then RT-PCR amplification was conducted with the designed TP gene sequence.
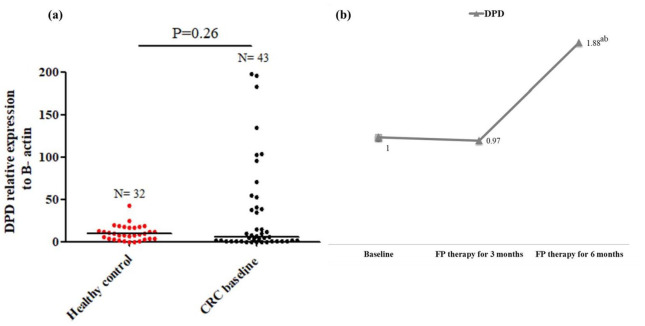
Baseline full methylation of DPD and the increase in DPD expression with FP therapy
The methylation status of DPD promoter was fully methylated (MDPD) in 95.3% our patients, and 60% of patients with MDPD showed significant downexpression of DPD (⩽4.26 folds), Table 4. The methylation status was not changed after FP therapy, as demonstrated in Figure 1. DPD expression in baseline CRC patients was insignificantly different compared with healthy controls, Figure 4a. After 6 months of FP therapy, DPD was significantly overexpressed by 2.88 folds, Figure 4b. Survival analysis showed significant reduction in the median EFS of patients with DPD expression >4.26 folds (median time = 14.63 vs median time = 20.83 months, P = 0.042), Figure 6. However, insignificant difference in the hazards of progression of our patients was observed between the low and high expression levels of DPD, Figure 7.
Figure 4.
DPD expression in healthy control and baseline CRC patients (a) and the change in DPD expression after 3 and 6 months of FP therapy normalised to their baseline levels (b).
aIs a significant difference when CRC patients after 3 and 6 months of FP therapy were compared with their baseline level, P value ⩽.05.
bIs a significant difference when CRC patients after 6 months of FP therapy were compared with their level after 3 months of FP therapy, P value ⩽.05.
Total RNA was extracted from the isolated lymphocytic cell pellets of healthy control and CRC patients at baseline and after 3 and 6 months of FP therapy. RNA was converted into cDNA, and then RT-PCR amplification was conducted with the designed DPD gene sequence.
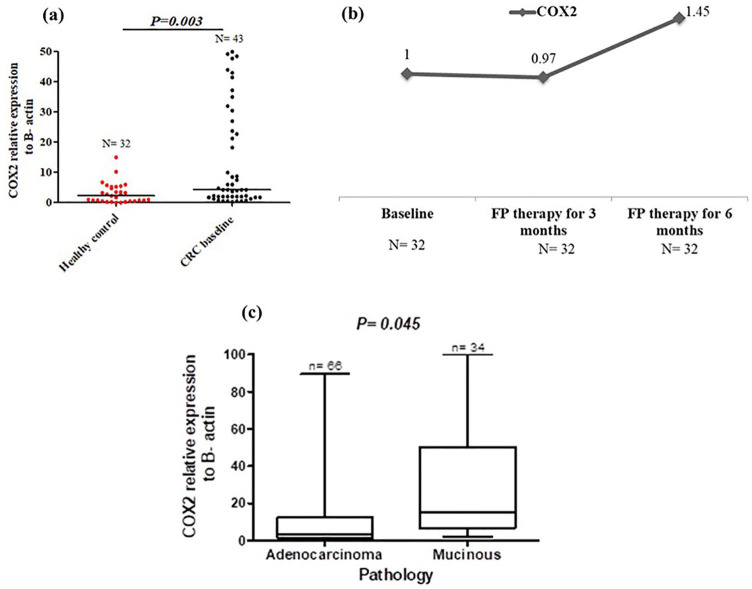
Baseline partial to full methylation with upregulation of COX2 and the increase in COX2 expression with FP therapy
The methylation status of COX2 promoter was partially methylated (UMCOX2) in 39.5% of patients, and fully methylated (MCOX2) in 55.8% of patients, Table 4. Almost 60% of patients with UMCOX2 and MCOX2 showed a signficant down-expression of COX2 level (⩽3.95 folds), Table 4. As the above 3 gene, no change was detected in the forms of COX2 methylation after therapy, Figure 1. The expression of COX2 was signficantly high in chemonaiive CRC patients (median = 16.97 vs median = 8.51 folds, P = 0.01) Figure 5a, and it was signficantly increased by 3.45 folds after 6 months of FP therapy, Figure 5b. Before FP therapy, the expression of COX2 was significantly high in patients with mucinous tumours (median = 7.88 vs median = 2.93 folds for adenocarcinoma patients, P = 0.045), Figure 5c. Kaplan-Meier analysis demonstrated in Figure 6 showed significant reduction in the median EFS of patients with COX2 expression >16.97 folds (median time = 14.78 months vs median time = 23.34 months for patients with COX2 expression ⩽16.97 folds, P = 0.022). Also the multivariate COX regression analysis, presented in Figure 7, revealed a significant increase in the hazard of progression associated with COX2 expression >16.97 fold (hazard ratio = 1.73, P = 0.050).
Figure 5.
COX2 expression in healthy control and baseline CRC patients (a), the change in COX2 expression after 3 and 6 months of FP therapy normalised to their baseline levels (b) and the COX2 expression in adenocarcinoma and mucinous tumours in baseline CRC patients (c).
Total RNA was extracted from the isolated lymphocytic cell pellets of healthy control and CRC patients at baseline and after 3 and 6 months of FP therapy. RNA was converted into cDNA, and then RT-PCR amplification was conducted with the designed COX2 gene sequence.
Discussion
In our previous research, we found that global DNA methylation had a significant impact on the treatment outcome with FP therapy in CRC patients.5 Monitoring the changes in the methylation status and the expression of FP metabolizing and COX2 genes over the period of FP therapy; was assumed to assist in the identification of CRC subgroups of patients who would benefit from the FP based treatment, and predict the response to therapy. So whole blood was our target site of investigation in order to undergo the molecular monitoring through a non-invasive sampling approach during the course of FP therapy. Moreover, it was demonstrated over literature that the degree of methylation in peripheral blood is almost similar to the level of DNA methylation in tumour tissue.17,18
Data of the present study illustrated that chemo-naïve CRC patients have unmethylation and overexpression of TS gene associated with high level of CA19.9, and mucinous tumour presentation, without effect in disease progression. In cancer, the expression and activity of TS protein are significantly related to cell doubling time; the faster the cell proliferation, the greater the expression and activity of TS.19,20 In harmony with our results, Kosuri et al.12 reported no evidence of methylation in TS gene promoter in mesothelioma cells. The correlation of the overexpression of TS gene with poor patients’ prognosis was assumed, in this study, to be through its correlation with CA19.9. It was presented in our previous article that our CRC patients with high CA19.9 showed significant deterioration in both overall survival and EFS regardless of tumour stage.5 In addition, Bai et al.21 considered the overexpression of TS gene as an adverse prognostic factor of CRC, correlated with bad tumour TNM classification and higher CRC staging. Moreover, its correlation with the mucinous type of CRC tumours was presented in Glasgow et al.22
In this study, FP therapy caused no changes in the methylation of TS gene promoter, while a significant induction of TS expression after therapy was observed. An association between high TS expression and resistance to 5-FU therapy was demonstrated in Gajjar et al.9 The overexpression of TS was linked to lack of DNA repair through DNA hypomethylation, and high microsatellite instability.23 The inactivation of TS gene with FP analogues, such as 5-FU resulted in depletion of thymidine pools, inhibition of DNA synthesis and subsequent cell death.24 Cell death stimulates TS gene for de novo synthesis of DNA. The stability of TS in ternary complexes and the reduction of free TS protein cause an induction of TS synthesis and synchronisation of cells into S-phase.24
The present study displayed full methylation of TP gene, in spite of its overexpression at baseline patients. With FP therapy, significant time dependent decrease in TP expression was observed and linked to CRC patients with good prognostic features such as patients at stage II of the disease, with right colon tumour and female sex. It has been evidenced that hypermethylation of gene promoter may be correlated with an increased, rather than a decreased level of expression in some of the genes, and the methylation at different regions of the gene sequence may have variable effects on its expression.25 In addition, the specific pattern of DNA methylation within the promoter region has a distinctive influence on gene expression which is tissue-specific.26
TP catalyses the reaction of thymidine to deoxyribose-1-phosphate and thymine. This deoxyribose is believed to have a role in the angiogenic effects of TP. In hypoxic tumour cells, TP activates cancer cells to secrete oxidative stress-angiogenic factors to promote angiogenesis, or it acts directly as an angiogenic factor.11 TP was found to be upregulated in tumour tissue of patients with advanced CRC. High TP expression was correlated with longer time to progression and related to the effect of treatment.27,28 On the other hand, TP has a great role in the metabolic activation of FP regimens, and the failure of capecitabine therapy was mainly attributed to lacking of TP enzymatic activity.12 The time-dependent decrease in TP activity in both tumour and normal adjacent tissues after treatment with 5-FU was attributed to the participation of TP enzyme in its own activation causing weakness in the required ‘useful’ effect of 5-FU.29,30
The contribution of methylation mechanism in the DPD deficiency was highlighted by Ezzeldin et al.13 In the current data, 60% of our baseline CRC patients with methylated DPD showed significant mRNA downexpression of DPD. After FP therapy, our CRC patients showed significant upregulation of DPD expression accompanied with significant reduction in their EFS. In the same way, Yu et al.31 reported DPD hypermethylation in CRC patients. However, the reduced DPD expression and activity levels were suggested to be tissue-specific irrespective to the promoter methylation.32,33 Higher DPD expression was associated metastatic CRC patients who received oxaliplatin based therapy in Baba et al.34 The overexpression of DPD confers resistance and non-responsiveness to 5-FU therapy.35 This resistance was suggested to be because of the deficiency of mismatch repair genes, which has been associated with DPD overexpression in CRC patients.36
The connection between COX2 expression and colorectal carcinogenesis was demonstrated since the discovery of the efficacy of non-steroidal anti-inflammatory drugs in the reduction of CRC risk in both humans and animal models.37 In this study, different forms of COX2 methylation (UCOX2, UMCOX2 and MCOX2) were shown. Down-expression of COX2 was significantly correlated with its promoter methylation. The median expression of COX2 was significantly high in the baseline CRC patients with mucinous tumours and at stage IV of the disease. COX2 expression was observed to be high in colonic adenocarcinomas compared to the adjacent normal mucosa.38 COX2 overexpression was found to be associated with loss of apoptosis, enhanced proliferation and stimulated angiogenesis through the activation of β-catenin/T cell factor, Ras and PI3K oncogenic signals in gastrointestinal epithelial cells.39 DNA methylation of COX2 gene was shown to be reduced in the blood of gastric cancer patients.40 Also, the level of methylation was seen to be not constant and continuously changing during the hepatocytic progression into hepatocellular carcinoma. That was proposed to be motivated by the silencing of tumour-suppressor genes and the activation of key oncogenes.41 The inconsistency in COX2 promoter methylation was also reported in both normal and colonic tumour tissues.14 So that, we suggest the dependance of COX2 expression on other extrinsic factors such as cytokines and tumour growth mediators along with the intrinsic dependance on its DNA promoter methylation.
COX2 expression was shown to be induced during the course of FP therapy received to our patients. Also a significant reduction in EFS was associated our CRC patients with high baseline COX2 expression. The elevated systemic inflammatory response associated with tumours, has been shown to be a risk factor for inferior survival in CRC patients.42 Moreover, it is known that chemotherapy induces mucosal damage through the increase of reactive oxygen species and proinflammatory cytokines such as IL1β, IL6 and TNFα.39 5-FU was claimed to increase myeloperoxidase activity in tissues, and proinflammatory cytokines in the sera of CRC patients.43
In this study the use of FP therapy did not change the methylation status of all genes. However, it significantly reduced the global level of 5-methylated cytosines in our previous work conducted on the same cohort of patients.5 That means, monitoring DNA methylation over the period of FP therapy is preferred to be estimated on global level using quantitative sensitive method of detection like mass spectroscopy rather than using qualitative method like MS-PCR. In the same way, the pattern of CpG islands methylation, and tumour suppressor genes expression were not consistently altered after treatment with DNA methyl transferase inhibitors in Mossman et al.44 and Flis et al.45 The effect on the DNA methylation pattern was suggested to happen after prolonged exposure to treatment in-vitro, and the effect on gene expression was suggested to be through other factors like proteins with a methyl-binding domain or histone modifications.44,45
In sum, 5-FU based therapy has a direct effect on the mRNA expression of its metabolizing genes and COX2 gene as well. However, its effect on their promoter’s DNA methylation detected with MS-PCR could not be confirmed. Also, the correlation between each gene promoter methylation and mRNA expression was found to be significant only with DPD and COX2, as significant % of CRC patients with downexpression of DPD and COX2 genes, showed DNA the full methylation state in the examined sequence of DPD and COX2 genes’ promoters. By the treatment with FP based therapy, significant induction in DPD and COX2 genes expression was detected, although a significant deterioration in the EFS was associated CRC patients with high baseline DPD and COX2 expression levels, and the hazard of progression was significantly associated patients with high baseline COX2 expression level. For TS gene which was unmethylated and overexpressed in baseline CRC patients. Its overexpression was associated patients with high baseline CA19.9, and mucinous tumour presentation. By the treatment with FP based therapy, significant induction in TS gene expression was detected. Also, TP gene was observed to be fully methylated and overexpressed in baseline CRC patients. By the treatment with FP based therapy, significant reduction in TP gene expression was detected especially in patients with good prognostic features.
In conclusion, close monitoring to the mRNA expression of FP metabolizing (TS, TP, DPD) and COX2 genes over the treatment period with 5-FU based therapy in the peripheral blood, will assist to predict the response to treatment for each CRC patient, as a good application for the trend of personalised therapy. Also, it will assist in avoiding disease progression through guiding the physician for metronomic dose adjustment,46 or implementing targeted therapy according to the genetic make-up of each patient.47 Undeniably, this study includes limitations of the limited number of patients, and the use of qualitative MS-PCR technique for the measurement of site-specific DNA methylation for correlation with genes mRNA expression. The quantitative analysis of site specific DNA methylation by pyrosequencing,48 or mass spectroscopy;49 will be a better investigational approach to apply pharmaco-epigenetics during the period of the patients’ follow-up in the clinic.
Acknowledgments
We thank all colleagues in Cancer Biology and Medical Oncology departments at Egyptian National Cancer Institute, Cairo University.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This research was supported by Egyptian National Cancer Institute, Cairo University.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Authors’ Contributions: MAF carried the experimental work, biostatistical analysis and drafting the manuscript. SES and MH extracted data of the patients, and shared in writing the manuscript. SAS, HFH and A-RZ shared in data analyses, writing and reviewing the manuscript. All authors have read and approved the manuscript.
Ethics Approval and Consent to Participate: This study was conducted according to Good Clinical Practice guidelines. The study was approved by the Institutional Human Research Ethics Committee of NCI, Egypt, number 00004025 and conducted in accordance with the Declaration of Helsinki and an informed consent was taken from each patient.
ORCID iD: Mariam Ahmed Fouad  https://orcid.org/0000-0003-0000-133X
https://orcid.org/0000-0003-0000-133X
References
- 1. Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055-6072. doi: 10.3748/wjg.v20.i20.6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers (Basel). 2013;5:676-713. doi:10.3390%2Fcancers5020676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:57-84. doi:10.1177%2F1758834015614530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ng JM, Yu J. Promoter hypermethylation of tumour suppressor genes as potential biomarkers in colorectal cancer. Int J Mol Sci. 2015;16:2472-2496. doi: 10.3390/ijms16022472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fouad MA, Salem SE, Hussein MM, et al. Impact of global DNA methylation in treatment outcome of colorectal cancer patients. Front Pharmacol. 2018;9:1173. doi:10.3389%2Ffphar.2018.01173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moon HH, Kim SH, Ku JL. Correlation between the promoter methylation status of ATP-binding cassette sub-family G member 2 and drug sensitivity in colorectal cancer cell lines. Oncol Rep. 2016;35:298-306. doi: 10.3892/or.2015.4342 [DOI] [PubMed] [Google Scholar]
- 7. Majchrzak-Celińska A, Baer-Dubowska W. Pharmacoepigenetics: an element of personalized therapy? Expert Opin Drug Metab Toxicol. 2017;13:387-398. doi: 10.1080/17425255.2017.1260546 [DOI] [PubMed] [Google Scholar]
- 8. Amatori F, Di Paolo A, Del Tacca M, et al. Thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase expression in colorectal cancer and normal mucosa in patients. Pharmacogenet Genomics. 2006;16:809-816. doi: 10.1097/01.fpc.0000230410.07899.bc [DOI] [PubMed] [Google Scholar]
- 9. Gajjar KK, Kobawala TP, Trivedi TI, et al. Influence of thymidylate synthase expression on survival in patients with colorectal cancer. Int J Adv Med Res. 2017;4:61-68. doi: 10.4103/IJAMR.IJAMR_32_17 [DOI] [Google Scholar]
- 10. Ju J. Beyond thymidylate synthase and dihydrofolate reductase: impact of non-coding microRNAs in anticancer chemoresistance. Curr Enzym Inhib. 2012;8:118-123. doi: 10.2174/157340812800793228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elamin YY, Rafee S, Osman N, Kenneth JO, Gately K. Thymidine phosphorylase in cancer; Enemy or friend? Cancer Microenviron. 2016;9:33-43. doi:10.1007%2Fs12307-015-0173-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosuri KV, Wu X, Wang L, Villalona-Calero MA, Otterson GA. An epigenetic mechanism for capecitabine resistance in mesothelioma. Biochem Biophys Res Commun. 2010;391:1465-1470. doi: 10.1016/j.bbrc.2009.12.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ezzeldin H, Lee A, Mattison L, Diasio R. Methylation of the DPYD promoter: an alternative mechanism for dihydropyrimidine dehydrogenase deficiency in cancer patients. Clin Cancer Res. 2005;11:8699-8705. doi: 10.1158/1078-0432.CCR-05-1520 [DOI] [PubMed] [Google Scholar]
- 14. Asting AG, Carén H, Andersson M, Lönnroth C, Lagerstedt K, Lundholm K. COX-2 gene expression in colon cancer tissue related to regulating factors and promoter methylation status. BMC Cancer. 2011;11:238. doi: 10.1186/1471-2407-11-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elzagheid A, Emaetig F, Alkikhia L, et al. High cyclooxygenase-2 expression is associated with advanced stages in colorectal cancer. Anticancer Res. 2013;33:3137-3143. [PubMed] [Google Scholar]
- 16. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG Islands. Proc Natl Acad Sci USA. 1996;93:9821-9826. doi: 10.1073/pnas.93.18.9821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barciszewska A-M, Nowak S, Naskręt-Barciszewska MZ. The degree of global DNA hypomethylation in peripheral blood correlates with that in matched tumor tissues in several neoplasia. PLoS One. 2014;9:e92599. doi: 10.1371/journal.pone.0092599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Wilson R, Heiss J, Breitling LP, Saum KU. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. doi:10.1186%2Fs13148-019-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu J, Schmitz JC, Lin X, et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochim Biophys Acta. 2002;1587:174-182. doi: 10.1016/s0925-4439(02)00080-7 [DOI] [PubMed] [Google Scholar]
- 20. Rose MG, Farrell MP, Schmitz JC. Thymidylate synthase: a critical target for cancer chemotherapy. Clin Colorectal Cancer. 2002;1:220-229. doi: 10.3816/CCC.2002.n.003 [DOI] [PubMed] [Google Scholar]
- 21. Bai W, Wu Y, Zhang P, Xi Y. Correlations between expression levels of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase, and efficacy of 5-fluorouracil-based chemotherapy for advanced colorectal cancer. Int J Clin Exp Pathol. 2015;8:12333-12345. [PMC free article] [PubMed] [Google Scholar]
- 22. Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259-264. doi:10.1038%2Fsj.bjc.6602330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gatalica Z, Vranic S, Xiu J, Swensen J, Reddy S. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Fam Cancer. 2016;15:405-412. doi: 10.1007/s10689-016-9884-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van der Wilt CL, Pinedo HM, Smid K, Peters GJ. Elevation of thymidylate synthase following 5-fluorouracil treatment is prevented by the addition of leucovorin in murine colon tumors. Cancer Res. 1992;52:4922-4928. [PubMed] [Google Scholar]
- 25. Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577-590. doi: 10.1016/j.ccr.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jang H, Shin W, Lee J, Do JT. CpG and non-CpG methylation in epigenetic gene regulation and brain function. Grayson DR, ed. Genes (Basel). 2017;8:148. doi:10.3390%2Fgenes8060148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindskog EB, Derwinger K, Gustavsson B, Falk P, Wettergren Y. Thymidine phosphorylase expression is associated with time to progression in patients with metastatic colorectal cancer. BMC Clin Pathol. 2014;14:25. doi: 10.1186/1472-6890-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Che J, Pan L, Yang X, et al. Thymidine phosphorylase expression and prognosis in colorectal cancer treated with 5-fluorouracil-based chemotherapy: a meta-analysis. Mol Clin Oncol. 2017;7:943-952. doi:10.3892%2Fmco.2017.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goto T, Shinmura K, Yokomizo K, et al. Expression levels of thymidylate synthase, dihydropyrimidine dehydrogenase, and thymidine phosphorylase in patients with colorectal cancer. Anticancer Res. 2012;32:1757-1762. [PubMed] [Google Scholar]
- 30. Stashkevych M, Khomutov E, Dumanskiy Y, Matviyenko A, Zinkovych I. The influence of 5-fluorouracil on activity of thymidine phosphorylase in gastric adenocarcinoma and normal adjacent tissue. Exp Oncol. 2014;36:267-270. [PubMed] [Google Scholar]
- 31. Yu J, McLeod HL, Ezzeldin HH, Diasio RB. Methylation of the DPYD promoter and dihydropyrimidine dehydrogenase deficiency. Clin Cancer Res. 2006;12:3864. doi: 10.1158/1078-0432.CCR-06-0549 [DOI] [PubMed] [Google Scholar]
- 32. Amstutz U, Farese S, Aebi S, Largiadèr CR. Hypermethylation of the DPYD promoter region is not a major predictor of severe toxicity in 5-fluorouracil based chemotherapy. J Exp Clin Cancer Res. 2008;27:54. doi: 10.1186/1756-9966-27-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Savva-Bordalo J, Ramalho-Carvalho J, Pinheiro M, et al. Promoter methylation and large intragenic rearrangements of DPYD are not implicated in severe toxicity to 5-fluorouracil-based chemotherapy in gastrointestinal cancer patients. BMC Cancer. 2010;10:470. doi: 10.1186/1471-2407-10-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baba H, Watanabe M, Okabe H, et al. Upregulation of ERCC1 and DPD expressions after oxaliplatin based first-line chemotherapy for metastatic colorectal cancer. Br J Cancer. 2012;107:1950-1955. doi: 10.1038/bjc.2012.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Negrei C, Hudita A, Ginghina O, et al. Colon cancer cells gene expression signature as response to 5-fluorouracil, oxaliplatin, and folinic acid treatment. Front Pharmacol. 2016;7:172. doi: 10.3389/fphar.2016.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Etienne-Grimaldi MC, Mahamat A, Chazal M, et al. Molecular patterns in deficient mismatch repair colorectal tumours: results from a French prospective multicentric biological and genetic study. Br J Cancer. 2014;110:2728-2737. doi: 10.1038/bjc.2014.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fischer SM, Hawk E, Lubet RA. Non-steroidal anti-inflammatory drugs and coxibs in chemoprevention: a commentary based primarily on animal studies. Cancer Prev Res (Phila.). 2011;4:1728-1735. doi:10.1158%2F1940-6207.CAPR-11-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14:1. doi: 10.1186/1471-230X-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melo ML, Brito GA, Soares RC, et al. Role of cytokines (TNF-a, IL-1b and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol. 2008;61:775-784. doi: 10.1007/s00280-007-0534-4 [DOI] [PubMed] [Google Scholar]
- 40. Su HJ, Zhang Y, Zhang L, et al. Methylation status of COX-2 in blood leukocyte DNA and risk of gastric cancer in a high-risk Chinese population. BMC Cancer. 2015;15 979. doi: 10.1186/s12885-015-1962-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen H, Cai W, Chu ES, et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene. 2017;36:4415-4426. doi: 10.1038/onc.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Proctor MJ, Talwar D, Balmar SM, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870-876. doi: 10.1038/sj.bjc.6605855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chang C-T, Ho TY, Lin H, et al. 5-Fluorouracil induced intestinal mucositis via nuclear factor-kB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7:e31808. doi: 10.1371/journal.pone.0031808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mossman D, Kim KT, Scott RJ. Demethylation by 5-aza-2’-deoxycytidine in colorectal cancer cells targets genomic DNA whilst promoter CpG island methylation persists. BMC Cancer. 2010;10:366. doi: 10.1186/1471-2407-10-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flis S, Gnyszka A, Flis K. DNA methyltransferase inhibitors improve the effect of chemotherapeutic agents in SW48 and HT-29 colorectal cancer cells. PLoS One. 2014;9:e92305. doi: 10.1371/journal.pone.0092305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fioravanti A, Canu B, Alì G, et al. Metronomic 5-fluorouracil, oxaliplatin and irinotecan in colorectal cancer. Eur J Pharmacol. 2009;619:8-14. doi: 10.1016/j.ejphar.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 47. Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tost J, Gut IG. Analysis of gene-specific DNA methylation patterns by Pyrosequencing® technology. In: Walker JM, Marsh S, eds. Pyrosequencing® Protocols. Methods in Molecular Biology™, vol. 373 Humana Press; 2007:89-102. doi: 10.1385/1-59745-377-3:89. [DOI] [PubMed] [Google Scholar]
- 49. Tost J, Schatz P, Schuster M, Berlin K, Gut IG. Analysis and accurate quantification of CpG methylation by MALDI mass spectrometry. Nucleic Acids Res. 2003;31:e50. doi: 10.1093/nar/gng050 [DOI] [PMC free article] [PubMed] [Google Scholar]