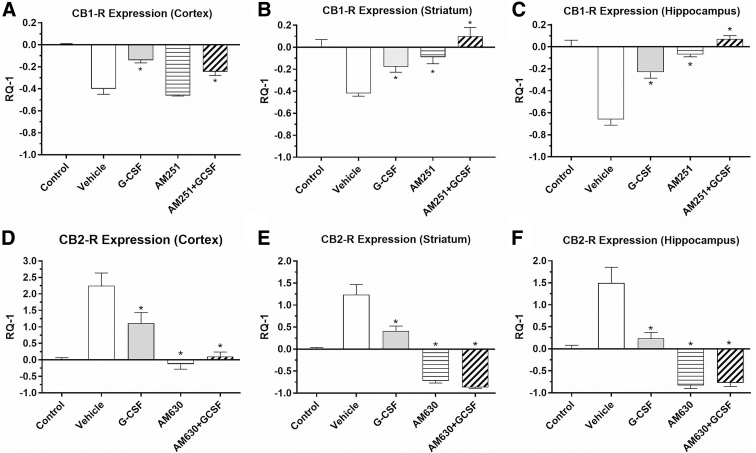
FIG. 1.
Effects of TBI on expression of CB1-R and CB2-R in brain regions (in right hemisphere, the side of injury), following 3 days of treatment with vehicle or G-CSF and CB-R antagonists. Data are expressed as mean RQ-1±SEM. Vehicle and G-CSF treatment groups utilized n=4 mice; CB-R antagonists (with and without G-CSF) groups utilized n=4 mouse samples per Rx group. (A–C) CB1-R mRNA was significantly downregulated in the three brain regions in vehicle-treated mice compared with controls without TBI; G-CSF treatment significantly reversed the effect on CB1-R downregulation in all regions compared with vehicle Rx (*p<0.05). Treatment with AM251 alone (CB1 receptor inverse agonist), 1 mg/kg i.p.×3 days, did not reverse downregulation in the cortex (A), but did reverse downregulation in the striatum (B) and hippocampus (C). The combination of AM251 with G-CSF enhanced the expression of CB1-R, reversing the downregulation of CB1-R caused by the TBI in all three regions. (D–F) CB2-R expression was significantly upregulated in all three regions in vehicle-treated mice compared with untreated control mice; treatment with G-CSF significantly reversed CB2 receptor upregulation in all three regions *p<0.05). Administration of the CB2-R antagonist (AM630), 1.0 mg/kg i.p.×3 days, alone significantly reduced CB2-R expression in all three regions. Coadministration of G-CSF with AM630 enhanced downregulation of CB2-R expression elicited by G-CSF treatment alone in all three regions. Statistical significance in each brain region was determined by one-way ANOVA, followed by the Dunnett multiple comparison test (comparison with vehicle treatment). *p<0.05. i.p., intraperitoneal; ANOVA, analysis of variance; SEM, standard error of mean; CB1-R, cannabinoid receptor 1; CB2-R, cannabinoid receptor 2; G-CSF, granulocyte colony-stimulating factor; TBI, traumatic brain injury.